Session Information
Date: Monday, October 27, 2025
Title: (0978–1006) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The rising incidence of connective tissue disease-associated interstitial lung disease (CTD-ILD) significantly worsens patient prognosis. While its pathogenesis remains unclear, immune-inflammatory dysregulation—particularly enhanced immune responses and impaired suppression—may drive chronic inflammation and fibrosis. T cells regulate fibroblast activation and collagen synthesis, interacting with other immune cells to accelerate fibrosis. Notably, reduced LAG3+ T cell populations in CTDs suggest iTreg dysfunction. Our prior studies identified elevated ATF3, a leucine zipper transcription factor, in pulmonary fibrosis. ATF3 may modulate inflammation, fibrosis, and immune responses. We hypothesize that ATF3 promotes fibrosis, and its knockdown could attenuate progression, possibly by expanding LAG3+ T cells.
Methods: 1. Establish a bleomycin-induced pulmonary fibrosis mouse model and measure ATF3 expression levels.2. Culture human lung fibroblasts in vitro and assess the effects of TGF-β1 induction on their proliferation, migration, and invasion capabilities.3. Construct siATF3 knockdown/overexpression in naive CD4+ T cells and examine their regulatory effects on lung fibroblast proliferation, migration, and invasion.4. Quantify LAG3+ T cell populations in peripheral blood of SSC-ILD patients using flow cytometry with dual-labeling techniques. Isolated LAG3+ T cells will be cultured, and their supernatants will be added to in vitro cultured human lung fibroblasts to evaluate fibrosis markers (F-actin and α-SMA).5. Evaluate spontaneous lung inflammation, collagen deposition, and LAG3+ T cell levels in ATF3-/- mice. Additionally, adoptive transfer of LAG3+ T cells from wild-type mice will be performed to assess their impact on lung pathology in bleomycin-induced pulmonary fibrosis models.
Results: 1. ATF3 expression is upregulated in bleomycin-induced pulmonary fibrosis mice.2. TGF-β1 induces overexpression of α-SMA, Smad, and ATF3 in human lung fibroblasts in vitro.3. ATF3 knockdown suppresses TGF-β1-induced proliferation, activation (myofibroblast transdifferentiation), and invasion of human lung fibroblasts.4. Peripheral blood LAG3+ T cell counts are reduced in SSC-ILD patients compared to controls.5. Supernatants from LAG3+ T cell cultures inhibit fibrotic marker expression (blocking TGF-β1-induced fibroblast-to-myofibroblast transition).Adoptive transfer of LAG3+ T cells ameliorates lung inflammation and fibrosis in murine models.
Conclusion: ATF3 promotes proliferation, migration, and invasion of lung fibroblasts, playing a critical role in pulmonary fibrosis progression. Knockdown of ATF3 significantly attenuates fibrotic progression, suggesting its utility as a therapeutic target. Adoptive transfer of LAG3+ T cells suppresses inflammatory responses and hyperproliferation of immune cells, demonstrating their protective role in fibrosis. These findings provide A novel strategy for treating CTD-ILD by targeting the ATF3/LAG3+ T cell axis, Translational potential for developing immunomodulatory or gene-based therapies.
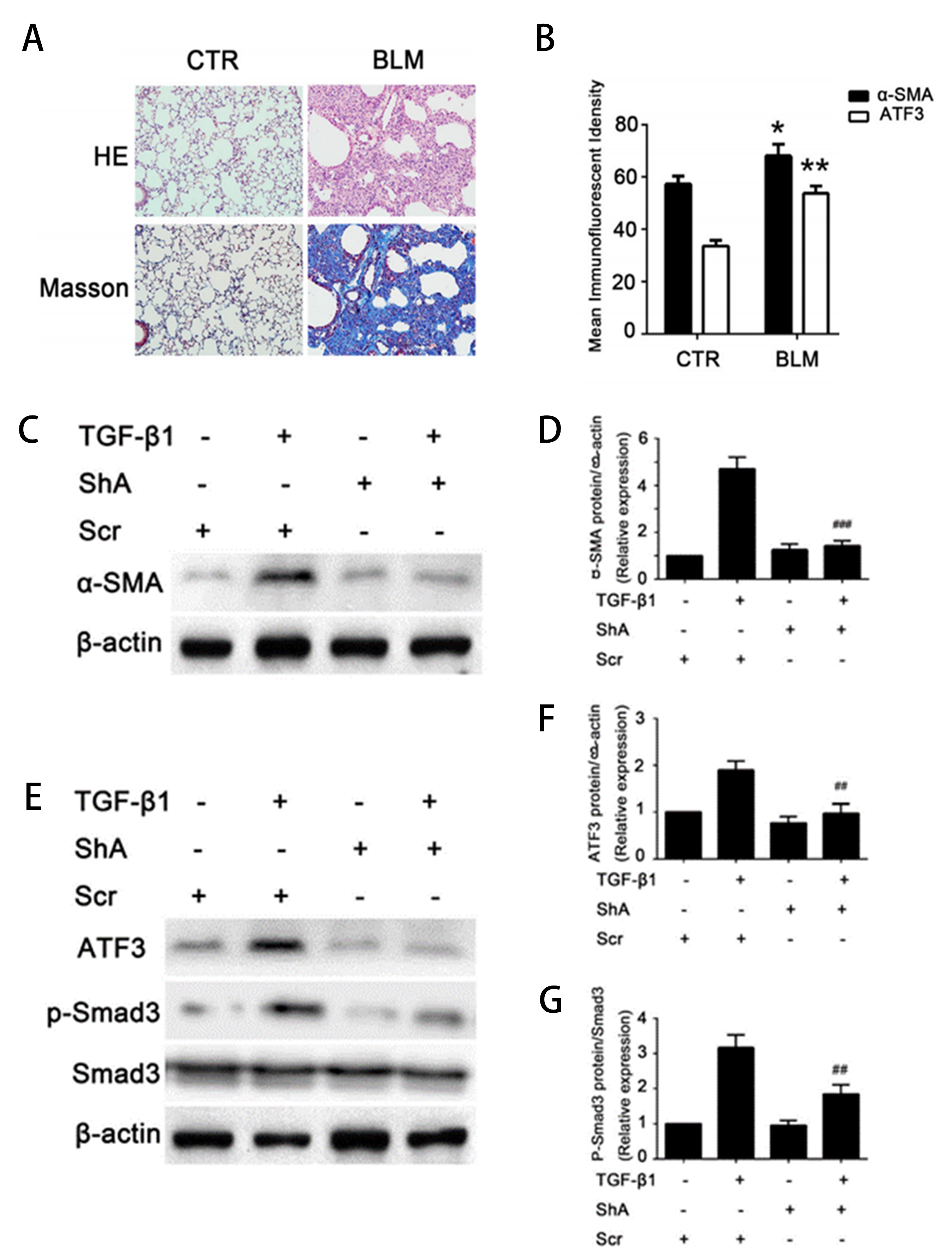 (A-B) Elevated expression of α-SMA and ATF3 in bleomycin-induced pulmonary fibrosis mouse model. (C-G) TGF-β1 induces ATF3 and Smad3 expression in human lung fibroblasts.
(A-B) Elevated expression of α-SMA and ATF3 in bleomycin-induced pulmonary fibrosis mouse model. (C-G) TGF-β1 induces ATF3 and Smad3 expression in human lung fibroblasts.
.jpg) (A-D) ATF3 knockdown suppresses TGF-β1-induced proliferation, myofibroblast differentiation, and invasion in human lung fibroblasts. (E-F) Significant reduction of CD4+CD25-LAG3+ regulatory T cells in peripheral blood of SSC-ILD patients.
(A-D) ATF3 knockdown suppresses TGF-β1-induced proliferation, myofibroblast differentiation, and invasion in human lung fibroblasts. (E-F) Significant reduction of CD4+CD25-LAG3+ regulatory T cells in peripheral blood of SSC-ILD patients.
.jpg) (A) Supernatants from LAG3+ Tregs inhibit TGF-β1-induced fibroblast-to-myofibroblast transition in human lung fibroblasts (20×) (Red: F-actin; Green: α-SMA; Blue: DAPI). (B) Adoptive transfer of LAG3+ T cells ameliorates pulmonary inflammation and fibrosis in murine models.
(A) Supernatants from LAG3+ Tregs inhibit TGF-β1-induced fibroblast-to-myofibroblast transition in human lung fibroblasts (20×) (Red: F-actin; Green: α-SMA; Blue: DAPI). (B) Adoptive transfer of LAG3+ T cells ameliorates pulmonary inflammation and fibrosis in murine models.
To cite this abstract in AMA style:
Zhang X, Qin l, Li Y. ATF3 Inhibits Pulmonary Fibrosis via CD4+CD25−LAG3+ T Cells [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/atf3-inhibits-pulmonary-fibrosis-via-cd4cd25%e2%88%92lag3-t-cells/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/atf3-inhibits-pulmonary-fibrosis-via-cd4cd25%e2%88%92lag3-t-cells/
