Session Information
Date: Monday, November 18, 2024
Title: SLE – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Anifrolumab, a monoclonal antibody targeting type I interferon (IFN-I) receptor subunit 1, was recently approved for the treatment of systemic lupus erythematosus (SLE) and has been shown successful particularly in patients with skin-predominant disease. Identification of a blood-based biomarker to identify those patients who respond to anifrolumab would provide a more personalized approach to care. Here, we used SIGLEC-1, a protein induced by IFN-I, to monitor patients treated with anifrolumab in a real-world cohort.
Methods: Patients with SLE (n=28) or cutaneous lupus (CLE, n=4) initiating therapy with anifrolumab were identified. Skin disease activity (CLASI-A), skin damage (CLASI-D), and systemic clinical activity (cSLEDAI) were collected at baseline (n=25, n=25, n=28 respectively) and longitudinally (n=22). Blood samples were collected longitudinally (6 mo) in a subset of patients (n=18) starting on day 1 of treatment and were used to quantify SIGLEC-1 expression on CD14+ monocytes by flow cytometry. We also measured by flow cytometry transitional B cells (CD38hi, CD27– B cells), a cell population strongly associated with IFN-I.
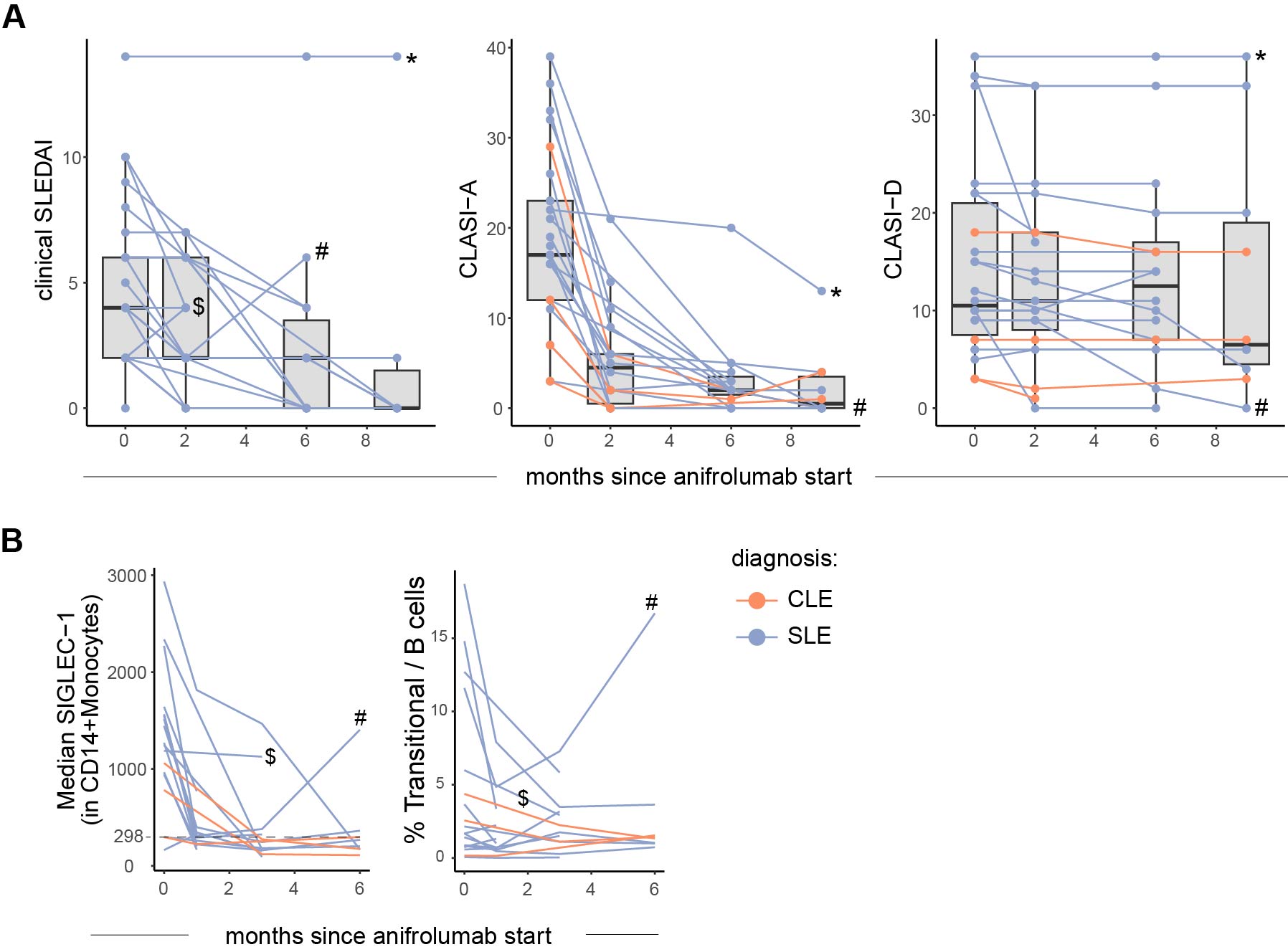
Results: Patients were mainly female (86%) and ancestrally diverse (non-Hispanic white = 38%), with median age 40 (IQR 31-54). Main indications for anifrolumab initiation were refractory mucocutaneous disease (n=29), joint symptoms (n=2), or fever (n=1). Most patients had severe, recalcitrant discoid lupus erythematosus (DLE, n=25); other cutaneous manifestations included subacute or acute cutaneous lupus, bullous lupus, or panniculitis. Median (IQR) baseline cSLEDAI, CLASI-A and CLASI-D were 4 (2-6), 17 (12-23) and 11 (8-21), respectively. Systemic and cutaneous disease activity improved in the majority of patients (both SLE and CLE) after anifrolumab initiation (Figure 1A). In patients with blood samples, baseline SIGLEC-1 correlated with cSLEDAI (rho=0.50, p=0.03), CLASI-A (rho=0.88, p< 0.001) and CLASI-D (rho=0.66, p=0.018). SIGLEC-1 decreased after anifrolumab initiation in most patients. Notably, two patients who did not show a reduction in SIGLEC-1 expression showed either no decrease (Patient-1) or a secondary increase (Patient-2) in SIGLEC-1 expression despite complete adherence (Figure 1B). Patient-1 had joint involvement at inclusion; anifrolumab was stopped after the second infusion due to lack of benefit and patient preference. Patient-2 had SLE with severe DLE refractory to belimumab. She showed a complete and rapid skin response early on, but developed synovitis and pericarditis during therapy (Figure 1B). In addition to an increase in type I IFN signature by SIGLEC-1, Patient-2 demonstrated a unique expansion in transitional B cells.
Conclusion: In this real-world cohort of lupus patients followed longitudinally after anifrolumab treatment, patients demonstrated improvement in both systemic and cutaneous manifestations. However, two patients with either refractory or relapsing disease after anifrolumab treatment demonstrated increased SIGLEC-1 expression coincident with disease relapse, highlighting the utility of SIGLEC-1 as a potential biomarker for monitoring patients.
To cite this abstract in AMA style:
Horisberger A, Shaw K, Dillon E, Marks K, Castillo R, Adejorin I, Hashemi K, Costenbader K, LaChance A, Vleugels R, Rao D. Associations Between Cytometric Interferon Signature and Clinical Response in a Cohort of Anifrolumab-treated Patients with Lupus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/associations-between-cytometric-interferon-signature-and-clinical-response-in-a-cohort-of-anifrolumab-treated-patients-with-lupus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-between-cytometric-interferon-signature-and-clinical-response-in-a-cohort-of-anifrolumab-treated-patients-with-lupus/