Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: SLE – Diagnosis, Manifestations, and Outcomes II: Complications
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Since the initial prospective validation of the Lupus Low Disease Activity State (LLDAS), this treat-to-target endpoint has been studied in numerous other cohorts, with results supporting its protective associations against flare and damage accrual. The mean duration of follow up in the original prospective validation was 2 years, potentially impacting on the ability to detect longer term signals in disease flare and irreversible damage, which typically accrues slowly. In this study we aimed to assess long-term associations of sustained LLDAS with protection from damage accrual and flare.
Methods: Adult SLE patients were recruited and followed prospectively from May 2013 to Dec 2020. Patients with ≥2 visits and no missing data were included in analysis. Multi failure time-to-event (Cox regression) analyses were used to assess the impact of sustained LLDAS on irreversible damage accrual (SLICC damage index) and flare (SELENA flare index), with dose and threshold effects studied. DORIS Remission was similarly assessed.
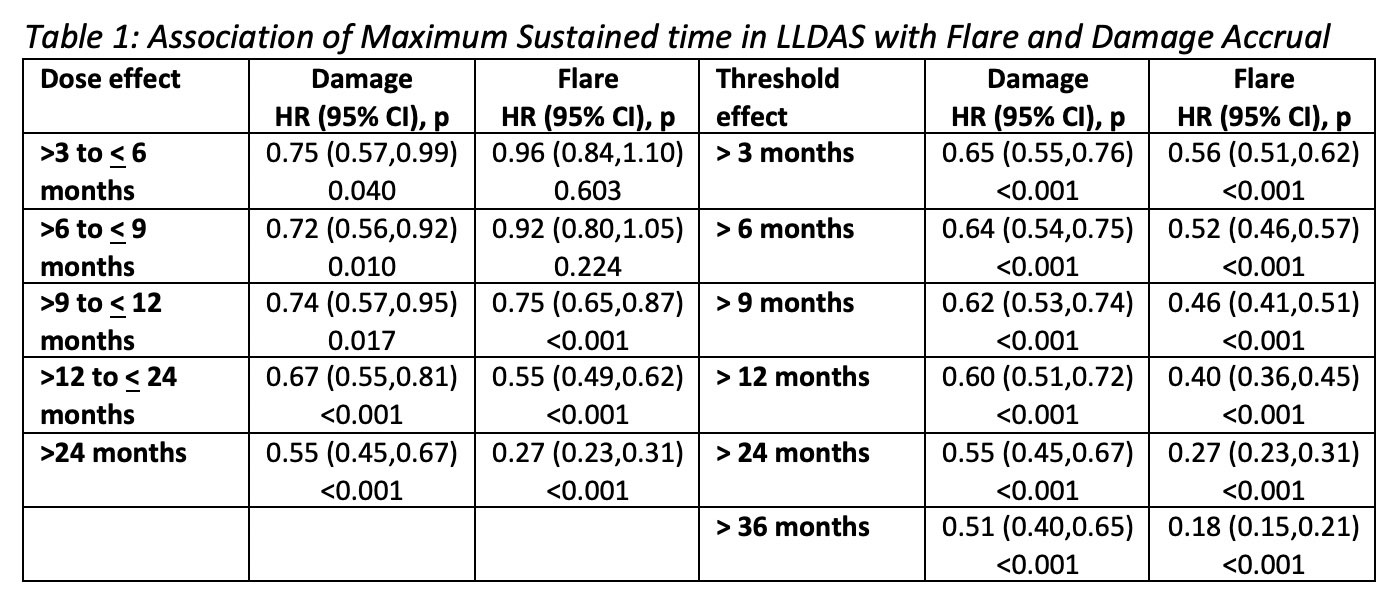
Results: 3,770 SLE patients were followed for (mean ± SD) 3.1 ± 2.4 years, totalling 37,834 visits. Most patients (n 3,128, 76.2%) attained LLDAS on at least one occasion. Any single visit in LLDAS was associated with significant protection against subsequent damage accrual (HR 0.60, 95%CI 0.52-0.69, p< 0.001) and flare (HR 0.57, 95%CI 0.53-0.61, p< 0.001). 2,699 (71.6%) sustained LLDAS for >3 months. Increasing durations of sustained LLDAS from >3 to >24 months corresponded to increased protective effects against damage and flare, whether measured as a dose effect or as a threshold (Table 1). LLDAS was more attainable compared to Remission (61.7% of patients ever), whilst conferring a similar magnitude of protection against damage and flare for sustained time ( >3 months Remission: HR 0.69, 95%CI 0.60-0.80, p< 0.001, and HR 0.65, 95%CI 0.60-0.71, p< 0.001 respectively).
Conclusion: In this long term prospective cohort study, we confirm significant protective effects of LLDAS against damage accrual and flare, as well as demonstrating deepening protection with longer durations of sustained LLDAS. LLDAS is a more attainable treat-to-target endpoint than remission while having similar protective effects.
Threshold effect – increasing threshold of sustained time;
Patients grouped based on maximum sustained time per patient
To cite this abstract in AMA style:
Golder V, Kandane-Rathnayake R, Li N, Louthrenoo W, Chen Y, Cho J, Lateef A, Hamijoyo L, Fen L, Wu Y, Navarra S, Zamora L, Li Z, Yuan A, Sockalingam S, Katsumata Y, Harigai M, Hao Y, Zhang Z, Basnayake D, Chan M, Kikuchi J, Takeuchi T, Bae S, Goldblatt F, Oon S, O'Neill S, Gibson K, Ng K, Law H, Tugnet N, Kumar S, Tee C, Tee M, Tanaka Y, Sing C, Hoi A, Nikpour M, Morand E. Association of Sustained Lupus Low Disease Activity State with Improved Outcomes in SLE: A Multinational Prospective Cohort Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/association-of-sustained-lupus-low-disease-activity-state-with-improved-outcomes-in-sle-a-multinational-prospective-cohort-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-sustained-lupus-low-disease-activity-state-with-improved-outcomes-in-sle-a-multinational-prospective-cohort-study/