Session Information
Date: Sunday, November 7, 2021
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Miscellaneous Aspects of RA (0786–0812)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: To evaluate baseline hemoglobin (Hb) and radiographic progression over time for patients in the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry.
Methods: BRASS is a prospective observational study of over 1,500 patients diagnosed with RA at the Robert Breck Brigham Arthritis Clinic in Boston, MA (NCT01793103). The study began enrollment in March 2003 and has a 4-year average follow up retention rate of 80%. An extended follow-up of up to 18 years is available for some patients. BRASS Hb data and total Sharp score (TSS) data fields were matched with the main BRASS subjects by subject ID and visit date (±15 days). Hb at baseline was categorized as low (Hb < 120 g/L for women or < 130 g/L for men) and normal (Hb ≥120 g/L for women or ≥130 g/L for men) as per WHO guidelines. The distributions of Hb at baseline overall and within current medication subgroups at baseline (anti-TNF, biologic DMARD, non-biologic DMARD) and demographics and disease characteristics overall and by low/normal Hb were summarized. Hb and TSS values and changes over time were described (overall, by low/normal Hb and current medication at baseline). All analyses were descriptive.
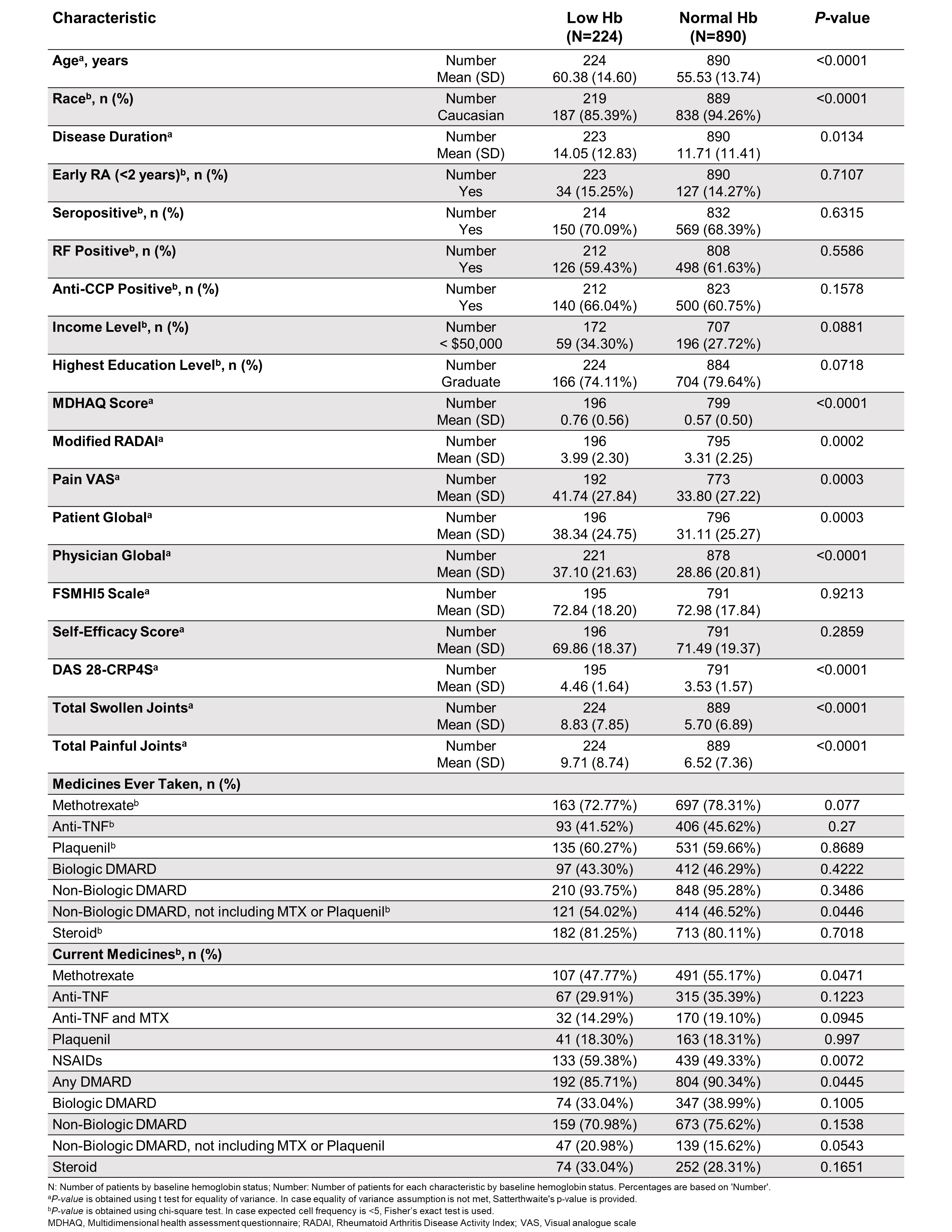
Results: Patients with low Hb (N=224) at baseline had longer disease duration, higher disease activity, reported more pain, had lower income levels, less education, and were less frequently Caucasian. Compared with patients with normal Hb, those with low Hb were less frequently MTX-experienced, on a combination of anti-TNF and MTX, or on a biologic DMARD at baseline; low Hb was more frequently associated with baseline use of non-biologic DMARD and NSAIDs (Table 1). Over the 10-year observation period patients with low Hb at baseline continued to have lower Hb than patients with normal Hb; although, on average, patients in the low Hb subgroup exhibited a steady increase in Hb levels. This increase in Hb was observed in all baseline treatment subgroups. A larger increase in TSS over time was observed for patients with low Hb than for patients with normal Hb. No meaningful differences potentially attributable to medication use at baseline were detected.
Conclusion: Patients with low Hb who were treated with anti-TNFs, biologic DMARDs, or non-biologic DMARDs experienced sustained improvements in Hb levels over time. Patients with lower Hb levels at baseline tended to have increased radiographic progression as measured by TSS compared to RA patients with normal Hb levels. Future analyses may evaluate constant therapy and non-constant therapy cohorts and disease severity cohorts.
To cite this abstract in AMA style:
Shadick N, Hagino O, Praestgaard A, Fiore S, Weinblatt M, Burmester G. Association of Hemoglobin Levels and Radiographic Progression in the BRASS Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/association-of-hemoglobin-levels-and-radiographic-progression-in-the-brass-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-of-hemoglobin-levels-and-radiographic-progression-in-the-brass-registry/