Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic inflammatory condition primarily affecting the joints and skin.1 More severe symptoms are associated with lower health-related quality of life, and many patients (pts) on recommended treatments (txs) achieve suboptimal responses.2,3 This study evaluated association between degree of joint and/or skin symptom resolution and improvements in patient-reported outcomes (PROs) among pts with PsA who initiated biologic or targeted synthetic (b/ts) DMARDs in the CorEvitas PsA/Spondyloarthritis Registry, a prospective, non-interventional United States research registry.
Methods: Eligible pts had a PsA diagnosis and initiated a b/tsDMARD on or after registry entry (first therapy in Registry) between April 2017–March 2025. Included pts had either body surface area (BSA) affected by psoriasis ≥3% or swollen joint count in 66 joints (SJC) ≥1 at initiation and were tx-persistent at follow-up 6 months (range: 5–9 months) post-initiation. Changes from initiation to follow-up in EuroQoL Visual Analog Scale (EQ-VAS) and pain VAS scores were each fit to linear regression models using BSA and SJC at follow-up as continuous exposure variables with their interaction, and using a categorical variable combining BSA and SJC with 6 levels: BSA=0% and SJC=0; BSA=0% and SJC ≥1; BSA >0% to < 3% and SJC=0; BSA >0% to < 3% and SJC ≥1; BSA ≥3% and SJC=0; BSA ≥3% and SJC ≥1. Results were summarized with counts, means, and standard deviation (SD) for continuous variables, and counts and percentages for categorical variables.
Results: Among 994 enrolled pts, mean age was 54.1 years; 581 (58.6%) were female, and 877 (89.8%) were white (Table 1). At tx initiation, mean (SD) BSA was 7.7% (13.4%) and mean (SD) SJC was 4.3 (6.0). At follow-up, BSA and SJC decreased to 2.4% (5.2%) and 1.9 (3.7), respectively. Mean EQ-VAS increased 5.0 points and pain decreased 12.9 points from initiation (Table 2). Compared with pts achieving optimal response at follow-up (235 [25.2%]; BSA=0% and SJC=0), pts who did not achieve optimal response had improvements in EQ-VAS with deficits ranging from −1.0 (BSA >0% to < 3% and SJC=0) to −8.4 (BSA ≥3% and SJC ≥1) and improvements in pain VAS with deficits ranging from −10.2 (BSA >0% to < 3% and SJC ≥1) to −15.8 (BSA ≥3% and SJC ≥1). Pts with BSA >0% to < 3% and SJC=0 at follow-up had minimal deficit in pain VAS improvement (Figure 1). Using continuous BSA and SJC, pts who had joint resolution without skin clearance, or had skin clearance without joint resolution, had deficits in EQ-VAS and pain VAS improvement compared with pts who achieved both at follow-up.
Conclusion: In this real-world cohort, optimal achievement of both joint resolution and skin clearance remained low 6 months after b/tsDMARD initiation and was associated with greater improvements in PROs compared with achieving only joint resolution, only skin clearance, or neither. These results highlight the importance of complete skin and joint symptom control for pts with PsA compared with focusing on either aspect alone.
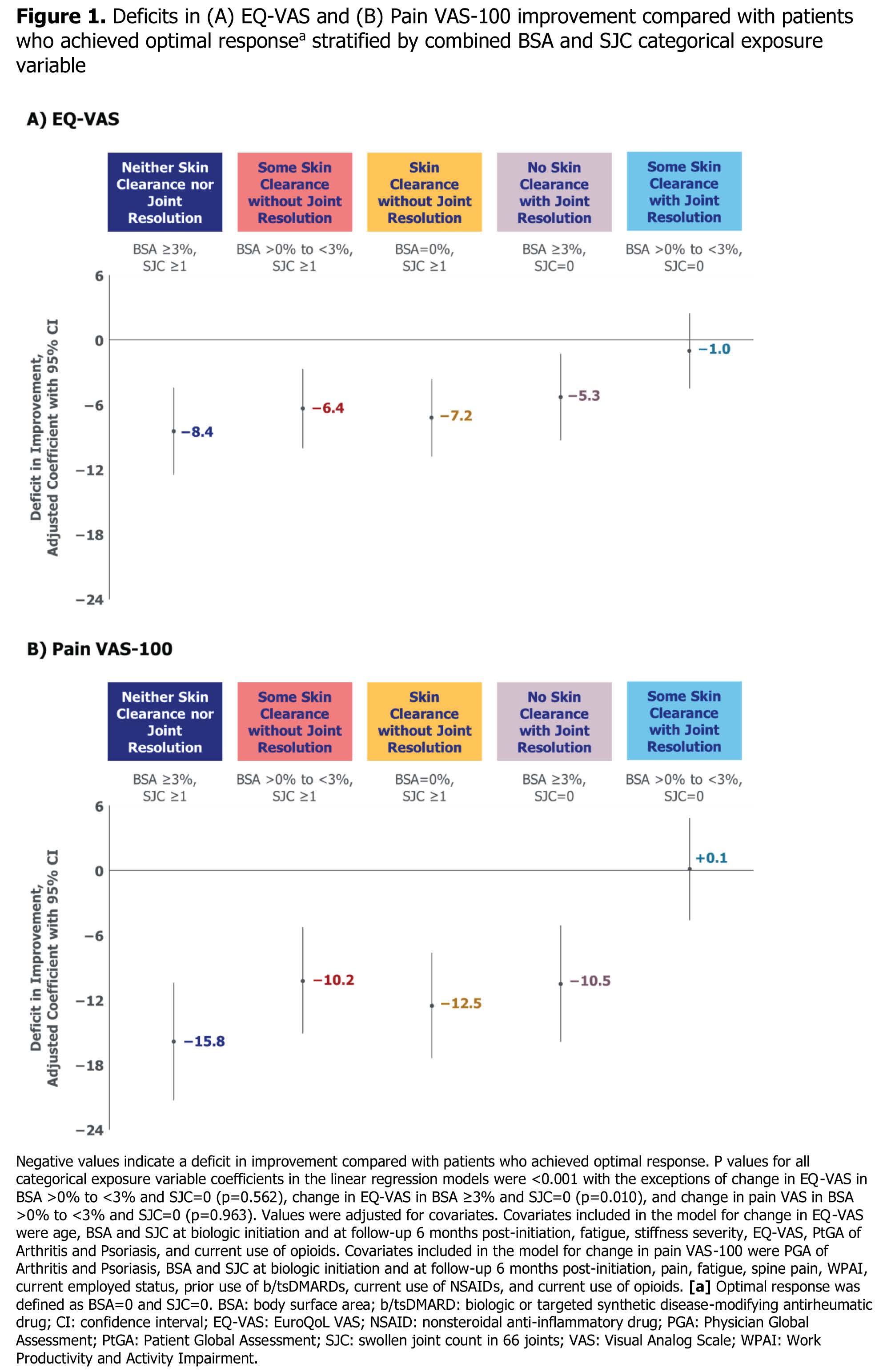 Table 1. Baseline demographic, lifestyle, and disease characteristics among patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry initiating a b/tsDMARD therapy
Table 1. Baseline demographic, lifestyle, and disease characteristics among patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry initiating a b/tsDMARD therapy
.jpg) Table 2. Disease activity and PRO measures at baseline and follow-up among patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry
Table 2. Disease activity and PRO measures at baseline and follow-up among patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry
.jpg) Figure 1. Deficits in (A) EQ-VAS and (B) Pain VAS-100 improvement compared with patients who achieved optimal response stratified by combined BSA and SJC categorical exposure variable
Figure 1. Deficits in (A) EQ-VAS and (B) Pain VAS-100 improvement compared with patients who achieved optimal response stratified by combined BSA and SJC categorical exposure variable
To cite this abstract in AMA style:
Mease P, Middaugh N, Muñoz Maldonado Y, Song C, Peterson S, Low R, Ogdie A. Association Between Skin and Joint Symptom Control and Patient-Reported Pain and Health Status Among Patients with Psoriatic Arthritis in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry Initiating Biologic or Targeted Synthetic Disease-Modifying Antirheumatic Drugs [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/association-between-skin-and-joint-symptom-control-and-patient-reported-pain-and-health-status-among-patients-with-psoriatic-arthritis-in-the-corevitas-psoriatic-arthritis-spondyloarthritis-registry-i/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-skin-and-joint-symptom-control-and-patient-reported-pain-and-health-status-among-patients-with-psoriatic-arthritis-in-the-corevitas-psoriatic-arthritis-spondyloarthritis-registry-i/
