Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Early predictors of response to treatment with upadacitinib (UPA), an oral Janus kinase inhibitor, could help to optimize a treat-to-target approach in patients with RA. This post hoc analysis evaluated whether early response to UPA treatment correlated with subsequent achievement of long-term clinical outcomes in patients with RA in whom ≥1 TNF inhibitor had previously failed.
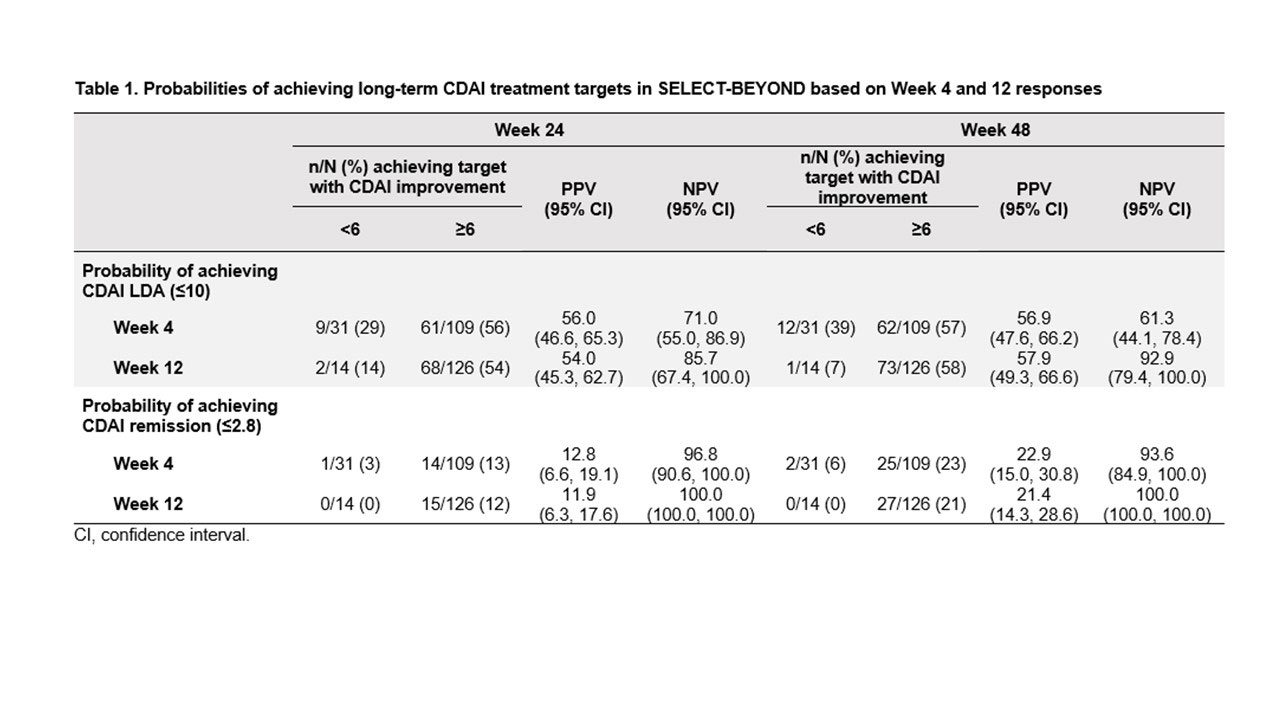
Methods: Data on patients who received UPA 15 mg once daily in whom ≥1 TNF inhibitor had failed (including patients with inadequate response or intolerance; TNFi-IR) were analyzed from two Phase 3 trials: SELECT-BEYOND and SELECT-CHOICE. Positive predictive value (PPV) and negative predictive value (NPV) were used to assess whether a ≥6-point improvement in Clinical Disease Activity Index (CDAI) and a ≥1.2-point improvement in DAS 28-joint count with CRP (DAS28-CRP) at Weeks 4 and 12 predicted achievement of CDAI low disease activity (LDA) or remission (≤10 or ≤2.8, respectively) and DAS28-CRP ≤3.2 and < 2.6, at Weeks 24 and 48 in SELECT-BEYOND, and at Week 24 in SELECT-CHOICE. Non-responder imputation was used for missing data.
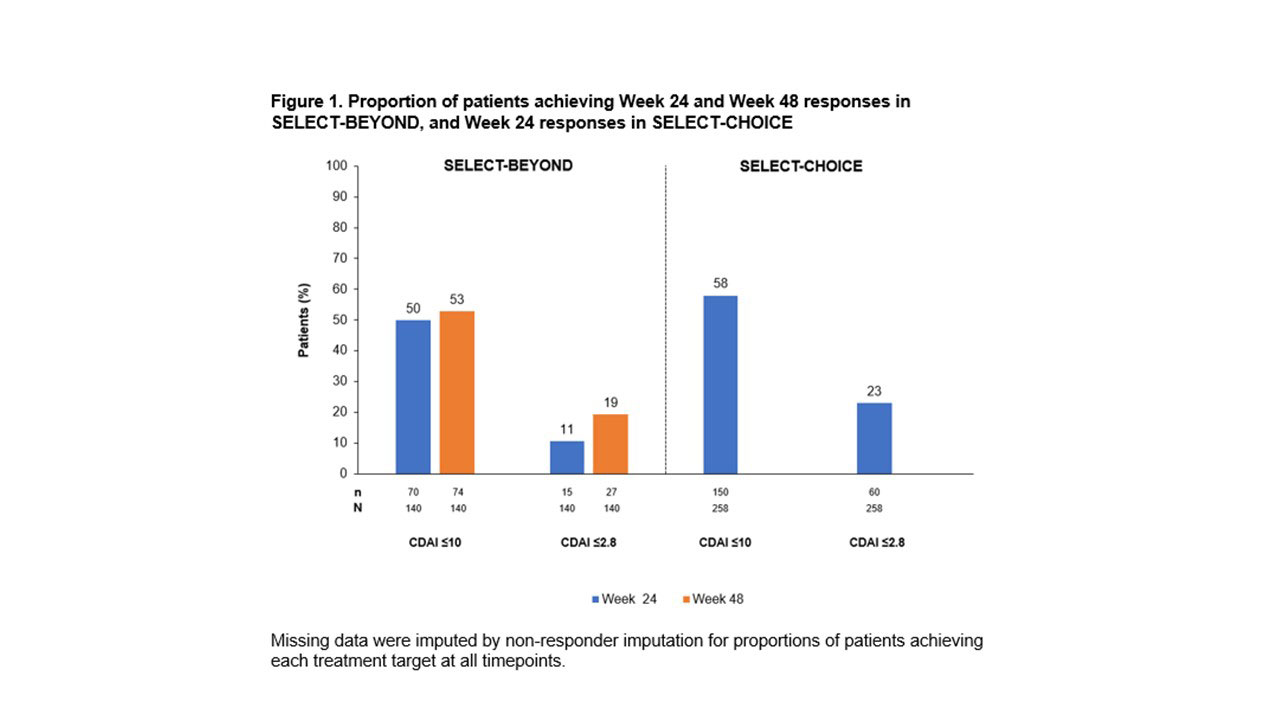
Results: The analysis included 140 TNFi-IR patients (89% inadequate response, 11% intolerant) from SELECT-BEYOND and 258 TNFi-IR patients (84% inadequate response, 16% intolerant) from SELECT-CHOICE. Across the studies, CDAI LDA and remission were achieved by 50–58% and 11–23% of patients, respectively (Figure 1). At Weeks 4 and 12 in SELECT-BEYOND, 31 (22%) and 14 (10%) patients, respectively, failed to achieve CDAI improvement ≥6 from baseline (Table 1). Of these, 9 (29%) and 2 (14%) patients achieved LDA, and 1 (3%) and 0 achieved remission at Week 24. The Week 24 NPVs for CDAI LDA were higher for Week 12 (86%) than Week 4 (71%); but similar for remission (97% [Week 4] and 100% [Week 12]). At Week 48, 12 patients (39%) and 1 patient (7%) without ≥6 CDAI improvement at Weeks 4 and 12, respectively, achieved CDAI LDA, while 2 (6%) and 0 achieved remission. The patterns and magnitude of the corresponding Week 48 NPVs were similar to those at Week 24 (Table 1). In SELECT-CHOICE, similar patterns to SELECT-BEYOND were seen at Week 24 in patients who did achieve CDAI improvement ≥6 from baseline (Table 2). Similar trends were observed when assessing the ability of DAS28 CRP improvement < 1.2/≥1.2 from baseline to Weeks 4 and 12 to predict DAS28 CRP ≤3.2 and < 2.6 treatment targets at Weeks 24 and 48 in SELECT-BEYOND and at Week 24 in SELECT-CHOICE.
Conclusion: Although most patients receiving UPA achieved early improvements in CDAI and DAS28-CRP scores, patients who did not demonstrate improvements of ≥6 in CDAI or ≥1.2 in DAS28-CRP within 12 weeks were unlikely to achieve disease activity targets with long-term treatment. Therefore, these patients may benefit from therapy adjustment, consistent with treat-to-target recommendations.
To cite this abstract in AMA style:
Charles-Schoeman C, Fleischmann R, Hall S, Kavanaugh A, Rubbert-Roth A, DeMasi R, Penn S, Garrison A, Anyanwu S, Sierra-Zorita R, Xavier R. Association Between Short-Term Response to Upadacitinib Treatment and Long-Term Clinical Outcomes in Patients with Rheumatoid Arthritis and Prior Inadequate Response to Tumor Necrosis Factor Inhibitor Therapy [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/association-between-short-term-response-to-upadacitinib-treatment-and-long-term-clinical-outcomes-in-patients-with-rheumatoid-arthritis-and-prior-inadequate-response-to-tumor-necrosis-factor-inhibitor/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-short-term-response-to-upadacitinib-treatment-and-long-term-clinical-outcomes-in-patients-with-rheumatoid-arthritis-and-prior-inadequate-response-to-tumor-necrosis-factor-inhibitor/