Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICIs), widely used to treat a variety of cancers, are associated with immune-related adverse events (irAEs) that are increasingly encountered in rheumatology clinics. However, it remains unclear how outcomes differ between patients with and without pre-existing rheumatoid arthritis (RA) receiving ICIs for cancer treatment. We aimed to compare irAE risk following ICI initiation between those with and without RA.
Methods: We conducted a population-based cohort study including patients receiving ICIs from 101 U.S. health care organizations between 2011 and 2025 in the U.S. We identified patients with pre-existing RA based on the presence of ≥2 ICD-10 codes before treatment with ICIs, including anti–programmed death-1/programmed death ligand-1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) agents. Propensity scores were calculated using demographics (age, sex, race/ethnicity), comorbidities, cancer type, metastatic status, prior cancer therapies, and previous RA treatments. Patients with RA receiving ICIs were matched 1:1 to ICI-treated patients without RA using propensity score matching. We then used Cox proportional hazards models to evaluate the risk of cutaneous, pulmonary, hepatic, and gastrointestinal irAEs among these two groups, overall and stratified by sex.
Results: We included 1,976 patients with RA and 1,976 propensity score-matched comparators (Table 1), with mean age 69 years, 55% female and 76% White. Lung cancer was the most common malignancy (57.1%) and 36.1% of patients with RA underwent radiation oncology treatment. After propensity score matching, those with RA had an 18% higher risk of cutaneous irAEs (aHR 1.18, 95% CI 1.01-1.39) and 17% higher risk of pneumonitis (aHR 1.17, 95% CI 1.01-1.36) versus those without RA (Table 2). When stratified by sex, females with RA had a higher risk than females without RA for cutaneous irAEs (aHR 1.26, 95% CI 1.02-1.57), colitis (aHR 1.32, 95% CI 1.03-1.70) and pneumonitis (aHR 1.36, 95% CI 1.10-1.68). There was no difference in the risks of these outcomes when comparing males with and without RA.
Conclusion: In this large nationwide study, those with pre-existing RA had a higher risk of cutaneous irAEs and pneumonitis. When stratifying by sex, females with RA had a higher risk of cutaneous irAEs, colitis and pneumonitis compared to females without RA while there was no difference by RA status among males. If future studies confirm these findings, these data can be used to counsel patients with RA contemplating starting an ICI as part of their cancer treatment.
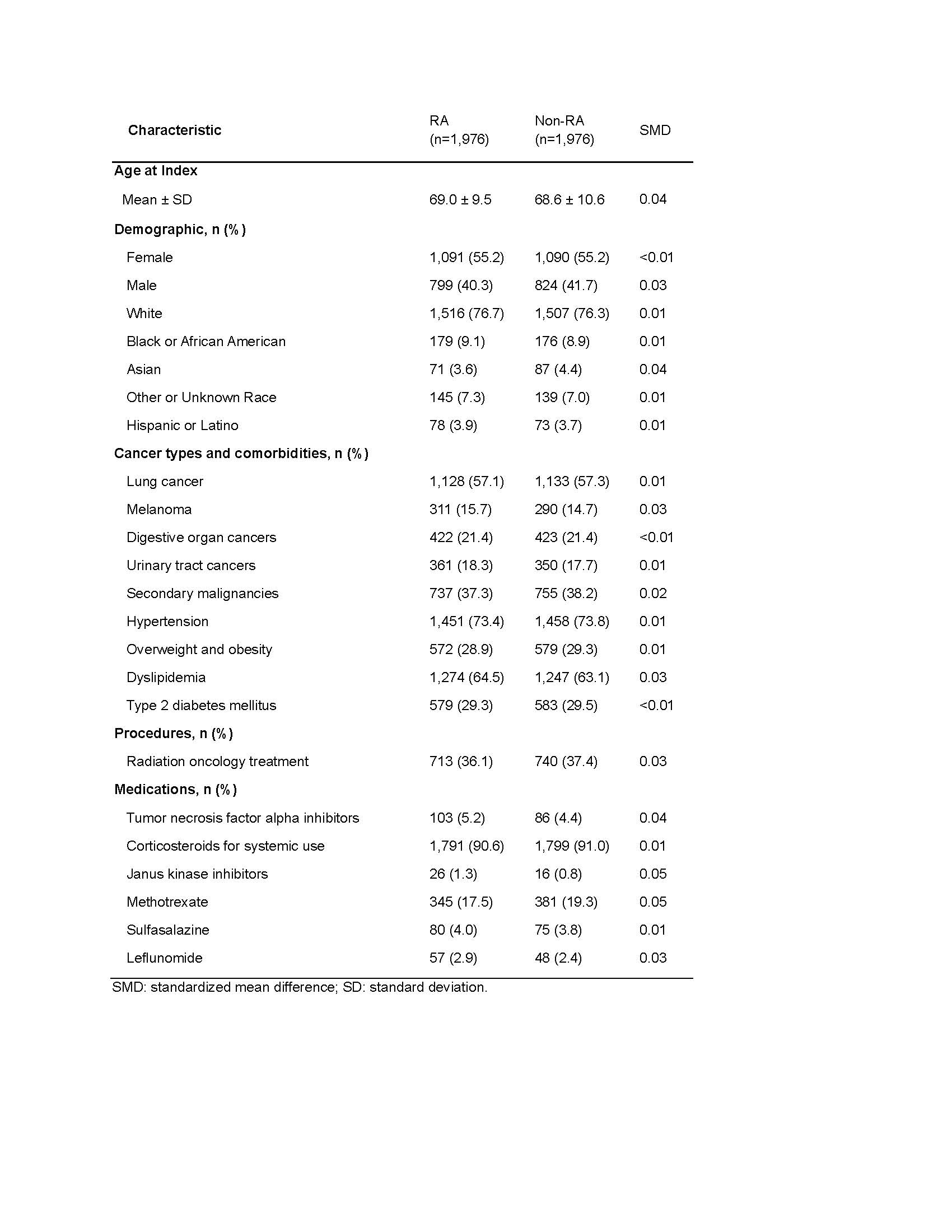 Table 1. Baseline characteristics of patients on immune checkpoint inhibitors with and without pre-existing rheumatoid arthritis (RA) after propensity score matching
Table 1. Baseline characteristics of patients on immune checkpoint inhibitors with and without pre-existing rheumatoid arthritis (RA) after propensity score matching
.jpg) Table 2. Effect of pre-existing rheumatoid arthritis on clinical outcomes among patients with cancer receiving immune checkpoint inhibitors after propensity score matching
Table 2. Effect of pre-existing rheumatoid arthritis on clinical outcomes among patients with cancer receiving immune checkpoint inhibitors after propensity score matching
.jpg) Table 3. Subgroup analysis stratified by sex, after propensity score matching
Table 3. Subgroup analysis stratified by sex, after propensity score matching
To cite this abstract in AMA style:
Ma K, Wu Y, Stovall R, Liew J, Singh N. Association between pre-existing rheumatoid arthritis and immune-related adverse events in patients with cancer receiving immune checkpoint inhibitors [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/association-between-pre-existing-rheumatoid-arthritis-and-immune-related-adverse-events-in-patients-with-cancer-receiving-immune-checkpoint-inhibitors/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-pre-existing-rheumatoid-arthritis-and-immune-related-adverse-events-in-patients-with-cancer-receiving-immune-checkpoint-inhibitors/
