Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with knee osteoarthritis are often overweight and suffer from comorbidities, which are frequently treated pharmacologically. Our understanding of the potential disease-modifying and/or symptom-modifying effects of commonly used pharmacological treatments is scarce, but recent studies have indicated beneficial effects of metformin, antihistamines, and vitamin D. The objective of this analysis was to investigate the effects of commonly used medications on changes in structural outcomes and WOMAC pain over a two-year period.
Methods: This is a post-hoc analysis of two large phase III trials investigating oral salmon calcitonin in knee OA (NCT00486434 and NCT00704847). The main inclusion criteria included American College of Rheumatology (ACR) criteria for diagnosis of knee OA and at least 150 out of 500 mm Western Ontario and McMaster Universities Osteoarthritis (WOMAC) knee pain at baseline. Eligible participants for the current study had knee x-rays with joint space width (JSW) and WOMAC pain questionnaires completed at baseline and two-year follow-up. Reported use of concomitant medication was grouped according to 3rd level ATC codes, and the duration of use was divided into the following categories: 1) no use, 2) 1-49 days, 3) 50-299 days, or 4) more than 300 days. The effects of the 13 most used medications on change in WOMAC pain and change in JSW from baseline to two years was evaluated using ANCOVA analysis, adjusting for baseline variables of age, sex, body mass index (BMI), and WOMAC pain/JSW.
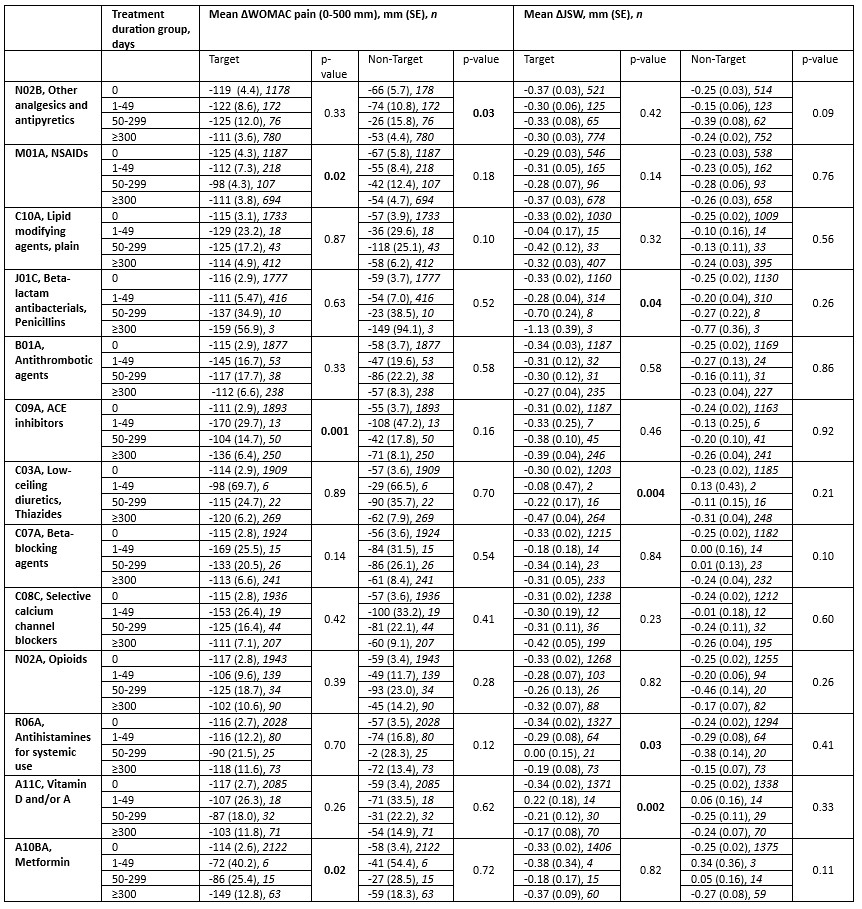
Results: A total of 2,206 participants were randomized in the trials. A reduction in target knee pain was associated with the use of metformin, with changes in the WOMAC pain score of -114, -72, -86, and -149 respectively for groups 1-4 (p=0.02), as well as ACE inhibitors, with changes in the WOMAC pain score of -111, -170, -104, and -136 respectively for groups 1-4 (p=0.001) (Table). Non-steroidal anti-inflammatory drug (NSAID) use was associated with a reduced improvement in pain over time (p=0.02). As previously reported, associations between the use of vitamin D, antihistamines, and thiazides with delayed structural progression were significant (p=0.002, p=0.03, and p=0.005, respectively). These findings, however, did not translate into a significant association with change in pain. Finally, paracetamol use was associated with a significant reduction in pain in the non-target knee (p=0.03).
Conclusion: Based on this exploratory analysis, commonly used medications in the OA knee population, such as metformin, NSAIDs, antihistamines, ACE inhibitors, vitamin D, and thiazides, may significantly impact structural and symptomatic changes over time. Further research is needed to substantiate the observed findings and to evaluate the underlying pathways that may mediate these associations.
To cite this abstract in AMA style:
Miller C, Baker M, Alexandersen P, Mondragón A, Adrian I, Karsdal M, Andersen J, Bihlet A. Association Between Knee Osteoarthritis Pain and Concomitant Drug Use: A Post-hoc Analysis of Two Phase 3 Clinical Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/association-between-knee-osteoarthritis-pain-and-concomitant-drug-use-a-post-hoc-analysis-of-two-phase-3-clinical-trials/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-knee-osteoarthritis-pain-and-concomitant-drug-use-a-post-hoc-analysis-of-two-phase-3-clinical-trials/