Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Dengue is the leading vectorborne disease globally with half of the global population at risk, including Puerto Rico in the United States. Previously, we showed that rheumatoid arthritis and related disease were associated with an increased risk of death after dengue diagnosis. Disease-modifying anti-rheumatic drugs (DMARDs), categorized into conventional-synthetic (cs) DMARDs, biological (b) DMARDs and target-synthetic (ts)DMARDSs, are standard therapy for patients with RA. Rituximab (RTX) therapy has been associated with higher COVID-19 mortality but outcomes associated with dengue have not been reported. The aim of this study was to assess the association between DMARDs and dengue outcomes.
Methods: The Taiwanese National Health Insurance Research Database (NHIRD), covering 99.5% of the Taiwan population was linked to the Notifiable Disease Dataset of Confirmed Cases (NDDCC) to assess associations of short-term dengue outcomes with RTX and other b/tsDMARDs. We identified 51,769 confirmed dengue cases aged ≥ 18 years from January 1, 2014 to December 31, 2015. Those cases were linked to the NHIRD to assess DMARDs use within one year before dengue was diagnosed and study outcomes of mortality and hospitalization within 30 days after dengue diagnosis. We categorized patients with dengue into four mutually exclusive groups based on prior use of DMARDs within one year: (1) RTX use, (2) use of b/tsDMARDs other than RTX ,(3) use of csDMARDs without use of b/tsDMARDs, (4) without use of any DMARDs. We examined the adjusted odds ratios (aORs) with 95% confidence intervals (CIs) for 30-day mortality and hospitalization in dengue patients based on prior use of DMARDs (csDMARDs users as reference) by multivariable logistic regression analysis adjusting for age, sex, socioeconomic status, year of diagnosis and comorbidities: RA and related disease, chronic kidney disease, diabetes, malignancy, coagulation and hemorrhagic disorders, coronary artery disease, hypertension, stroke, congestive heart failure, chronic obstructive pulmonary disease, asthma, major depressive disorder, and liver cirrhosis.
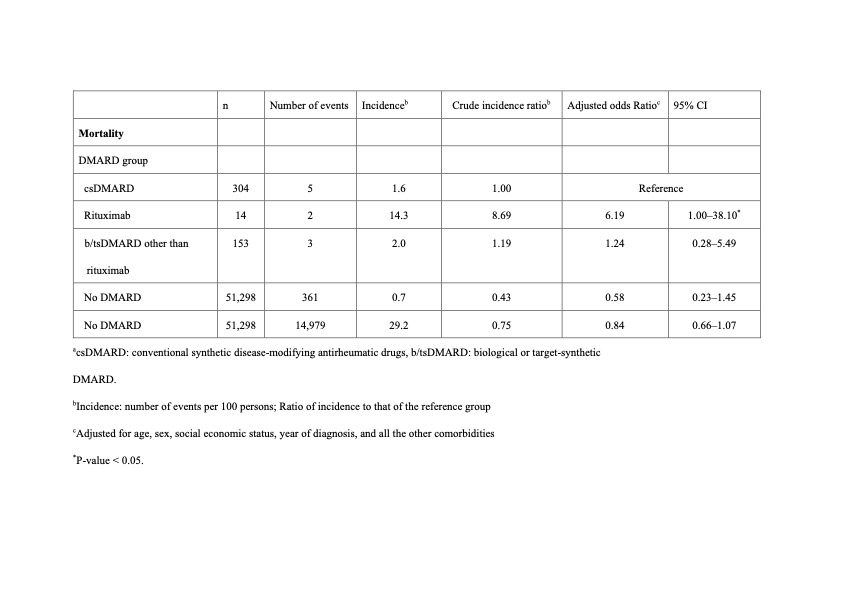
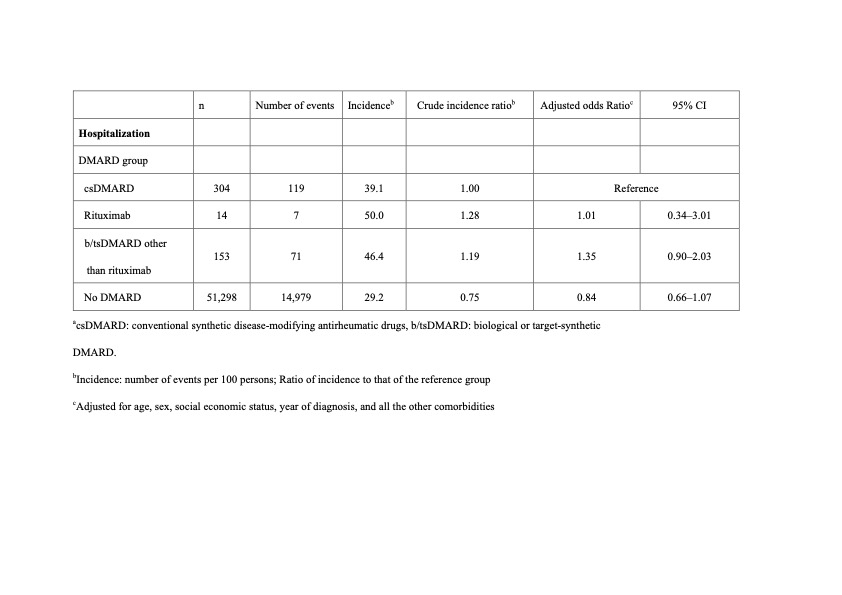
Results: Table 1 shows the numbers and incidences of death within 30 days after dengue diagnosis. Compared with patients treated with csDMARDs only, risk of 30-day mortality was higher in patients with prior rituximab use (aOR, 6.19; 95% CI, 1.00–38.1; p < 0.05), but not in patients receiving b/tsDMARDs other than RTX. However, the risk of 30-day hospitalization was not significantly different between patients receiving csDMARDs treatment and patients with prior use of RTX or other b/tsDMARDs (Table 2).
Conclusion: This population-based study showed that use of rituximab was associated with a higher risk of death within 30 days after dengue diagnosis compared with use of csDMARDs.
To cite this abstract in AMA style:
Lin I, Chen H, Tsai T, Huang N. Association Between Disease-modifying Anti-rheumatic Drugs and Short-term Outcomes of Dengue: A Population-based, Cohort Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/association-between-disease-modifying-anti-rheumatic-drugs-and-short-term-outcomes-of-dengue-a-population-based-cohort-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-disease-modifying-anti-rheumatic-drugs-and-short-term-outcomes-of-dengue-a-population-based-cohort-study/