Session Information
Date: Tuesday, November 9, 2021
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes (1909–1914)
Session Type: Abstract Session
Session Time: 2:15PM-2:30PM
Background/Purpose: The achievement of disease control has been shown to be associated with improved prognosis in PsA, though no single measure of low disease activity or remission is currently universally accepted. Patient-reported outcomes (PROs) have been well-established in PsA and are important indicators of patient improvement while on treatment. To date, the association between PROs and disease control in PsA has not been fully characterized. We examined the association between clinically meaningful improvement in PROs and stringent measures of disease control among patients with PsA enrolled in the Phase 3 SELECT-PsA 1 trial.
Methods: Patients with active PsA and an inadequate response to ≥1 non-biologic DMARDs were randomized to receive upadacitinib (UPA) 15 mg once daily (QD), UPA 30 mg QD, adalimumab (ADA) 40 mg every other week, or PBO for 24 weeks. PROs included: Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), 36-Item Short-Form Health Survey (SF-36), and Work Productivity and Activity Impairment (WPAI). Measures of stringent disease control included achievement of minimal disease activity (MDA), ACR70 response, and remission based on Disease Activity Index in PsA (DAPSA ≤4.0) or PsA Disease Activity Score (PASDAS ≤1.9). The percentage of patients achieving stringent disease control was determined among patients reporting vs not reporting PRO improvements ≥ minimal clinically important differences (MCID) in the combined active treatment and PBO group at Week 24.
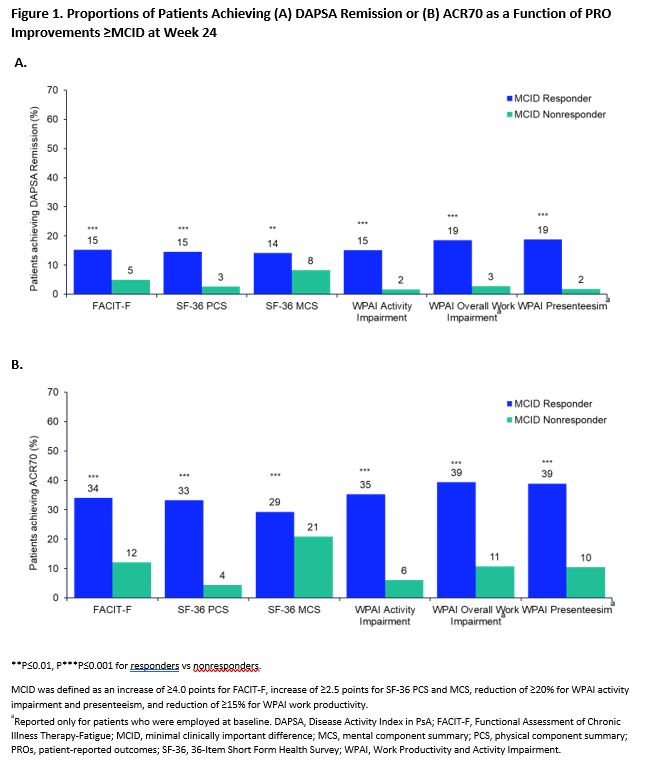
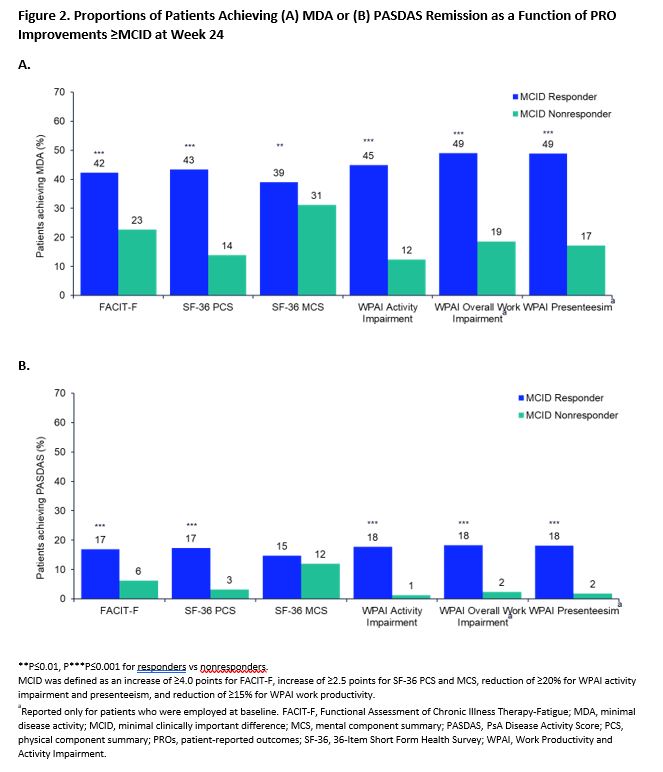
Results: A total of 1704 patients were included in the SELECT PsA 1 trial, of whom 59.2%, 72.4%, 51.3%, 62.3%, 64.6%, and 63.9% reported improvements ≥ MCID (MCID responders) in FACIT-F, SF-36 physical component summary score, SF-36 mental component summary (MCS) score, WPAI activity impairment, WPAI overall work impairment, and WPAI presenteeism, respectively, at week 24. The percentage of patients achieving MDA, ACR70 or DAPSA remission at week 24 was significantly higher (nominal P≤0.01) among patients who reported improvements ≥ MCID for all PROs vs those who did not (Figures 1,2). Similar results were seen in patients achieving PASDAS remission except for SF-36 MCS score (Figure 2). Among patients reporting improvements > MCID across all PROs, more patients achieved ACR70 and MDA responses (29%-49%) with fewer patients achieving DAPSA or PASDAS remission (14%-19%).
Conclusion: PsA patients who reported clinically meaningful improvements in key PROs: fatigue, quality of life, and work productivity were more likely to achieve stringent measures of disease control. These results suggest a close association between meaningful improvements in patient-centric outcomes and achievement of stringent disease control.
To cite this abstract in AMA style:
Gossec L, Damjanov N, Tsuji S, Lertratanakul A, Lippe R, Patel J, Zueger P, de Vlam K. Association Between Clinically Meaningful Improvements in Patient-Reported Outcomes and Stringent Measures of Disease Activity in Patients with Psoriatic Arthritis Treated with Upadacitinib versus Placebo or Adalimumab: Results from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/association-between-clinically-meaningful-improvements-in-patient-reported-outcomes-and-stringent-measures-of-disease-activity-in-patients-with-psoriatic-arthritis-treated-with-upadacitinib-versus-pla/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-clinically-meaningful-improvements-in-patient-reported-outcomes-and-stringent-measures-of-disease-activity-in-patients-with-psoriatic-arthritis-treated-with-upadacitinib-versus-pla/