Session Information
Date: Sunday, November 7, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Measurements (0739–0763)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Back pain is the hallmark disease feature for patients (pts) with AS. However, the relationship between back pain and other patient-centric outcomes and disease activity measures has not been well characterized. The purpose of this study was to examine the association between meaningful back pain improvement and quality of life, disease activity, physical functioning, fatigue, sleep, and work productivity in pts with AS treated with upadacitinib (UPA) or placebo (PBO) in the phase 2/3 SELECT-AXIS 1 trial1.
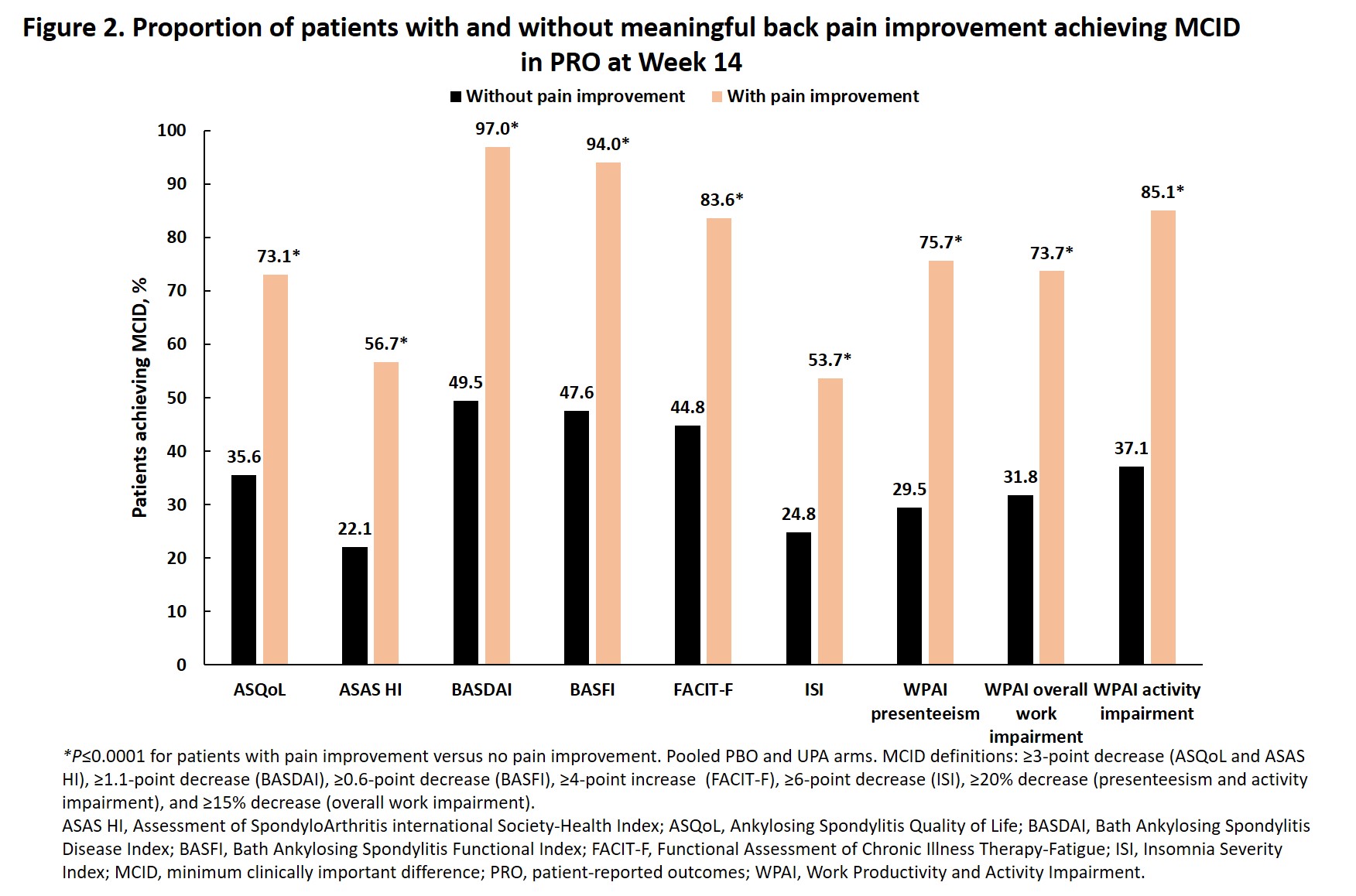
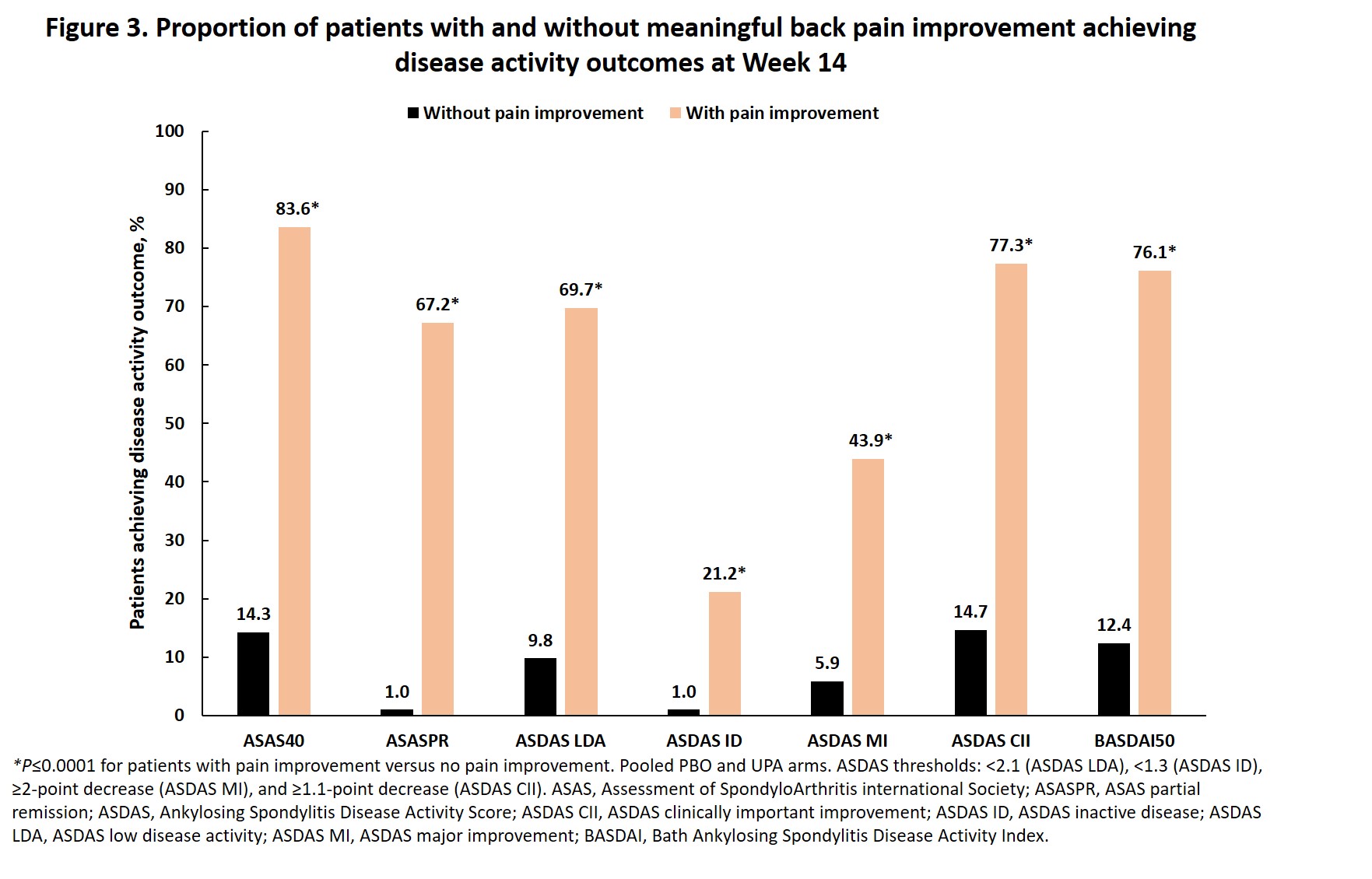
Methods: Active AS pts with an inadequate response to NSAID therapy received UPA 15 mg once daily or PBO for 14 weeks, followed by a 90-week open label extension period. Clinically meaningful back pain improvement was defined as achieving a score of < 4 and ≥2-point decrease from baseline in Patient's Assessment of Total Back Pain score on a numeric rating scale and was assessed at all time points up to Week 52. The proportions of pts maintaining Week 4 meaningful back pain improvement at Week 14 and the proportion of pts at Week 14 in the pooled UPA and PBO arms reporting values ≥ minimum clinically important differences (MCID) in patient-reported outcomes (PROs: BASFI, BASDAI, BASFI, Assessment of SpondyloArthritis international Society-Health Index [ASAS HI], AS Quality of Life [ASQoL], Functional Assessment of Chronic Illness Therapy – Fatigue [FACIT-Fatigue], Insomnia Severity Index [ISI] scores, and Work Productivity and Activity Impairment [WPAI; presenteeism, overall work impairment, and activity impairment]), and achieving disease activity outcomes (ASAS40, ASAS partial remission, AS Disease Activity Score [ASDAS; low disease activity, inactive disease, major improvement, and clinically important improvement], and BASDAI50) as a function of meaningful back pain improvement were also assessed. Analyses were performed using either the Cochran-Mantel-Haenszel test, adjusting for high-sensitivity C-reactive protein with non-responder imputation for comparison between treatments, or Chi-Square test with as observed analysis for comparison between responders/non-responders. P-values < 0.05 were nominal.
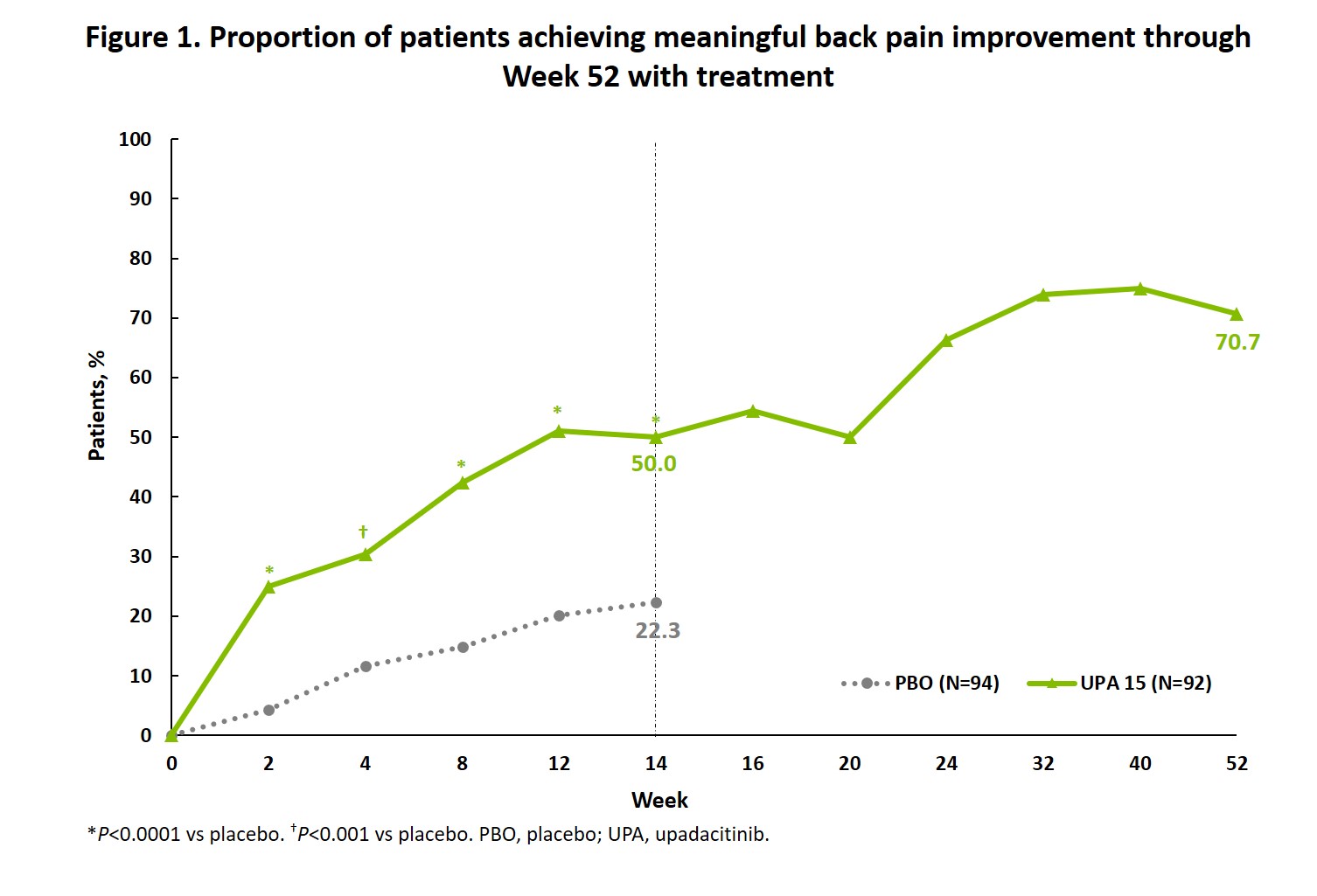
Results: Meaningful improvement in back pain was achieved by a significantly greater proportion of pts with AS receiving UPA versus PBO starting at Week 2 (P < 0.001), with further improvements in the UPA group between Week 14 and Week 52 (Figure 1). Of pts achieving back pain improvement at Week 4, 92.9% of pts on UPA 15 compared to 54.5% of pts on PBO maintained this response at Week 14 (P < 0.05). A significantly greater proportion of pts who attained meaningful back pain improvement at Week 14 vs those who did not reported values ≥MCID in all PROs and had greater response across all disease activity outcomes (P≤0.0001, Figures 2 and 3).
Conclusion: A significantly greater proportion of pts with AS achieved meaningful improvement in back pain with UPA compared to PBO starting at Week 2, with continued improvement through 52 weeks of treatment. Meaningful back pain improvement was associated with meaningful improvement in other PROs and achievement of important measures of AS disease activity.
Reference: 1. Van der Heijde et al. Lancet. 2019;394:2108–2117
To cite this abstract in AMA style:
Baraliakos X, Bessette L, Salvarani C, Chen N, Lippe R, Patel J, Song I, Zueger P, Goupille P. Association Between Clinically Meaningful Back Pain Improvement and Patient-reported Outcomes and Disease Activity in Patients with Ankylosing Spondylitis: Results from a Phase 2/3 Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/association-between-clinically-meaningful-back-pain-improvement-and-patient-reported-outcomes-and-disease-activity-in-patients-with-ankylosing-spondylitis-results-from-a-phase-2-3-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-clinically-meaningful-back-pain-improvement-and-patient-reported-outcomes-and-disease-activity-in-patients-with-ankylosing-spondylitis-results-from-a-phase-2-3-trial/