Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In a US national observational study conducted in a clinical practice setting, patients who were positive (+) for anti-cyclic citrullinated peptide (CCP) antibodies at baseline showed a greater clinical response to treatment with abatacept (ABA), but not an anti-TNF inhibitor (TNFi), compared with those who were anti-CCP negative (–).1 This study assessed whether baseline anti-CCP status was associated with response to ABA or non-TNFi in patients with RA.
Methods: Patients with RA from the Corrona registry, aged ≥18 years, who initiated ABA, rituximab (RTX), tocilizumab (TCZ) or tofacitinib (TOF) from 12/1/2005–2/28/2019 were included. Eligible patients had to have anti-CCP measures at/prior to index date (ABA or non-TNFi initiation date), must never have used ABA prior to index date and had to have 6 months’ follow-up after index date. Patient characteristics at index were compared by anti-CCP status (+, ≥20 U/mL; –, < 20 U/mL). Outcomes included changes in CDAI (primary), modified HAQ and patient global assessment (PGA) from baseline to 6 months, and proportion of patients achieving LDA or remission, minimal clinically important difference in CDAI and modified ACR response at 6 months. Predicted mean differences were estimated using mixed-effects linear regression models adjusting for baseline covariates (if p< 0.1) with site as a random effect; odds ratios were estimated using a mixed logistic regression model with the anti-CCP– group as a reference and site as a random effect. Responses for ABA, RTX, TCZ and TOF were estimated separately; comparison of responses to ABA and specific non-TNFi were estimated within a similar time period of initiation (2006, 2010 or 2012).
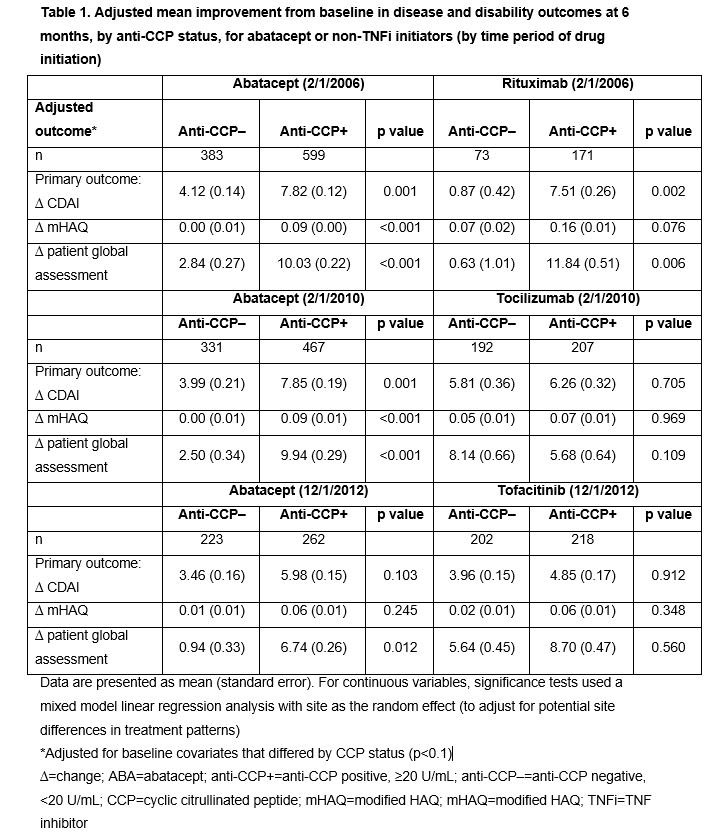
Results: Overall, 982 ABA, 399 TCZ, 244 RTX and 420 TOF initiators were identified. Across treatments, anti-CCP+ (vs anti-CCP–) patients had longer disease duration and were more likely to have erosive changes on X-ray; additionally, a higher percentage were RF+ and more were in ACR functional class III–IV at index. Association of response at 6 months by anti-CCP status is shown in Tables 1 and 2. During most time periods, adjusted mean changes in CDAI, modified HAQ and PGA were significantly higher for anti-CCP+ vs anti-CCP– patients for ABA (Table 1). For ABA-treated patients, the odds of achieving most secondary outcomes were significantly higher for anti-CCP+ vs anti-CCP– groups (Table 2). Adjusted mean change in CDAI and PGA (Table 1) and odds of achieving LDA were significantly higher (Table 2) in anti-CCP+ vs anti-CCP– groups for RTX-treated patients; differences in other secondary outcomes were observed but were not statistically significant (Tables 1 and 2). No significant differences were seen by anti-CCP status for TCZ- and TOF-treated patients. Limitations included sample size and relatively short length of treatment time.
Conclusion: In patients treated with abatacept and RTX, but not TCZ or TOF, those who were anti-CCP+ had a greater clinical response than anti-CCP– patients at 6 months’ follow-up post index.
Reference:
- Harrold LR, et al. J Rheumatol 2018;45:32–9.
Professional medical writing and editorial assistance was provided by Catriona McKay PhD, at Caudex, and was funded by Bristol-Myers Squibb.
To cite this abstract in AMA style:
Harrold L, Shan Y, Rebello S, Guo L, Connolly S, Zhuo J, Kelly S, Lehman T. Association Between Baseline Anti-citrullinated Protein Antibody Status and Response to Abatacept or Non-TNF Inhibitor Therapy in Patients with RA: Results from a US National Observational Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/association-between-baseline-anti-citrullinated-protein-antibody-status-and-response-to-abatacept-or-non-tnf-inhibitor-therapy-in-patients-with-ra-results-from-a-us-national-observational-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-baseline-anti-citrullinated-protein-antibody-status-and-response-to-abatacept-or-non-tnf-inhibitor-therapy-in-patients-with-ra-results-from-a-us-national-observational-study/