Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Pharmacotherapy, including non-steroidal anti-inflammatory agents (NSAIDs), is commonly used to alleviate symptoms of osteoarthritis (OA); however, these medications may increase the risk of adverse effects and drug interactions, especially in older adults taking multiple medications for other comorbid conditions. Accurate documentation of medication use in OA research is important and is often based on self-report. While self-reported medication use provides insights into real-world patterns of medication use, its validity in research studies remains largely unknown. In this study, we evaluated the validity of the Osteoarthritis Initiative’s (OAI) medication inventory form by comparing self-reported use with metabolites or active ingredients measured in plasma.
Methods: The OAI had a structured protocol to collect detailed information on prescription medications. Participants were told to bring all medications used in the 30 days before each study visit. The study staff then recorded all the prescription medications. If a participant forgot to bring their medications, the interviewer relied on a medication list or conducted a follow-up call to complete the medication inventory form. Although drug metabolites and active ingredients were not routinely measured in the OAI, a case-cohort sub-study that included 465 participants had plasma assayed using liquid chromatography-tandem mass spectrometry. We calculated sensitivity, specificity, and kappa statistics for the most commonly reported medications with metabolite or active ingredient plasma levels.
Results: Among the 465 participants, 301 (65%) were females, 197 (42%) had frequent knee pain, and the mean age was 60 years (SD=9) with a mean body mass index of 27.8 kg/m2 (SD=4.6). Table 1 shows strong agreement between self-report and presence of metabolites for antihypertensive medications (e.g., hydrochlorothiazide κ=0.71) and anti-diabetic medications (e.g., metformin κ=0.97), with sensitivity exceeding 85% and specificity exceeding 90%. For NSAIDs, we found strong agreement for celecoxib (κ=0.94), but not naproxen (κ=0.42) or ibuprofen (κ=0.23). While specificity was high for celecoxib, naproxen, and ibuprofen ( >97%), sensitivity was high only for celecoxib.
Conclusion: The validity of self-reported medication use in the OAI varied by medication. Sensitivity and agreement were high for some medications but lower for others. The three medications with low sensitivity were the only ones we examined that were available over the counter at the baseline visit (2004-2006). Specificity was high across all medications. These findings highlight the need to consider potential misclassification for specific drug classes when using the data from the OAI medication inventory, as some classes offer more reliable and higher accuracy of self-reported medication use than others.
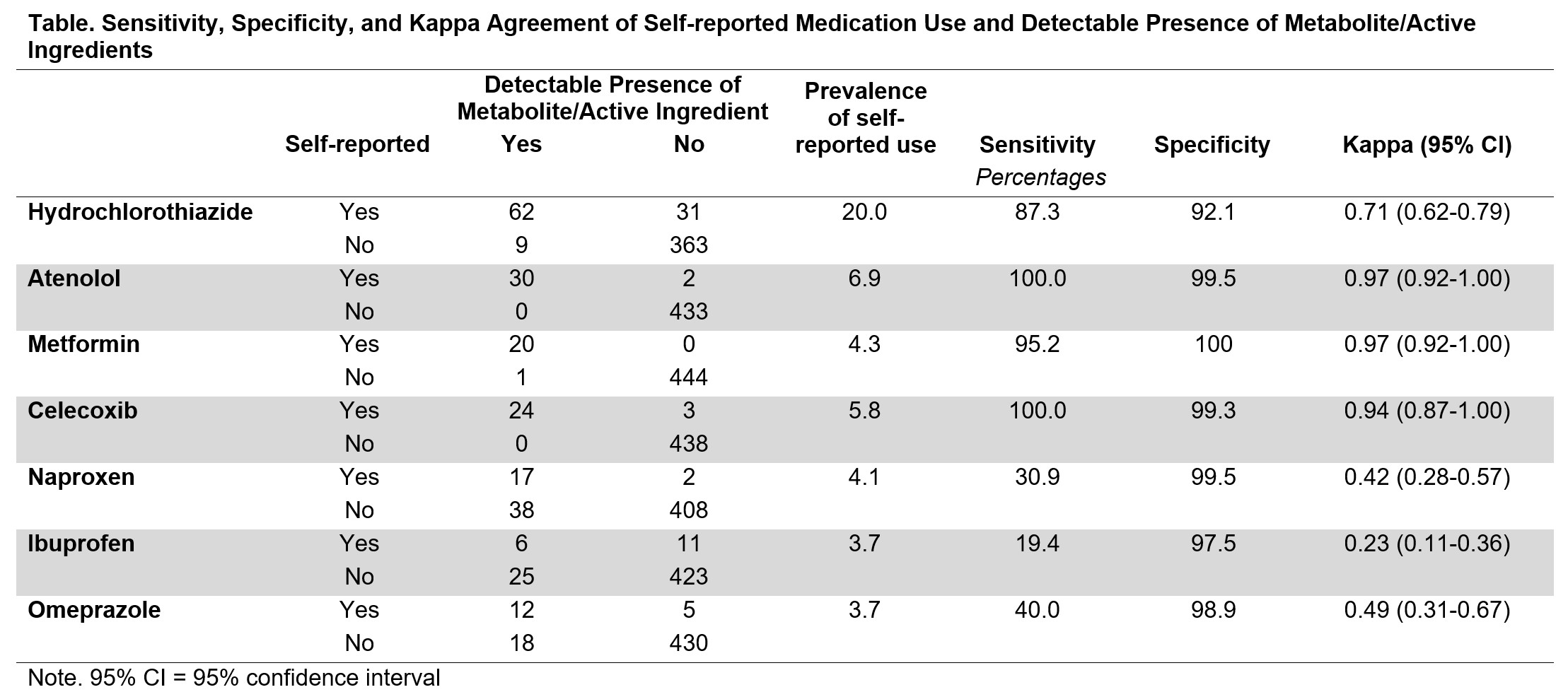 Table. Sensitivity, Specificity, and Kappa Agreement of Self-reported Medication Use and Detectable Presence of Metabolite/Active Ingredients
Table. Sensitivity, Specificity, and Kappa Agreement of Self-reported Medication Use and Detectable Presence of Metabolite/Active Ingredients
Disclosures: K. Lapane: None; A. Hume: None; J. Driban: None; S. Liu: None; T. McAlindon: Anika, 2, Grunenthal, 2, Kiniksk, 2, Kolon TissueGene, Inc., 2, Medipost, 2, Novan, 2, Organogenesis, 2, Regeneron, 2, Remedium-Bio, 2, Samumed, 2, Sanofi, 2, Scarcell, 2, Visor, 2; C. Eaton: None; S. Xu: None; B. Lu: None.
To cite this abstract in AMA style:
Lapane K, Hume A, Driban J, Liu S, McAlindon T, Eaton C, Xu S, Lu B. Assessing the Validity of Self-Reported Medication Data Through Metabolite Analysis: Data from the Osteoarthritis Initiative [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/assessing-the-validity-of-self-reported-medication-data-through-metabolite-analysis-data-from-the-osteoarthritis-initiative/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-the-validity-of-self-reported-medication-data-through-metabolite-analysis-data-from-the-osteoarthritis-initiative/
