Session Information
Date: Tuesday, November 12, 2019
Title: 5T112: Metabolic & Crystal Arthropathies II: Genetics & Physiology (2834–2839)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Hyperuricemia is common in patients with type 2 diabetes mellitus and is associated with an increased risk of gout. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, the newest class of medications for adults with diabetes mellitus, promote glycosuria and also lower serum uric acid levels through urinary excretion. The objective of our study was to assess the risk of gout among adults prescribed an SGLT2 inhibitor versus a glucagon-like peptide-1 receptor (GLP1) agonist.
Methods: We conducted a population-based new-user cohort study from March 2013 to December 2017 using claims data from a US nationwide commercial insurance database (IBM MarketScan). Patients with type 2 diabetes mellitus newly prescribed an SGLT2 inhibitor were 1:1 propensity score-matched to patients newly prescribed a GLP1 agonist. Patients were excluded if they had a history of gout or had received gout-specific treatment previously. The primary outcome was new diagnosis of gout based on an inpatient diagnosis of gout or the combination of an outpatient diagnosis and use of a gout-related medication within 14 days thereafter. The rate of heart failure hospitalization (positive control based on the known effect of SGLT2 inhibitor on reducing heart failure) and cellulitis (negative control) were also assessed. Cox proportional hazards regression was used to estimate hazard ratios (HR) of the primary outcome and 95% confidence intervals (CI).
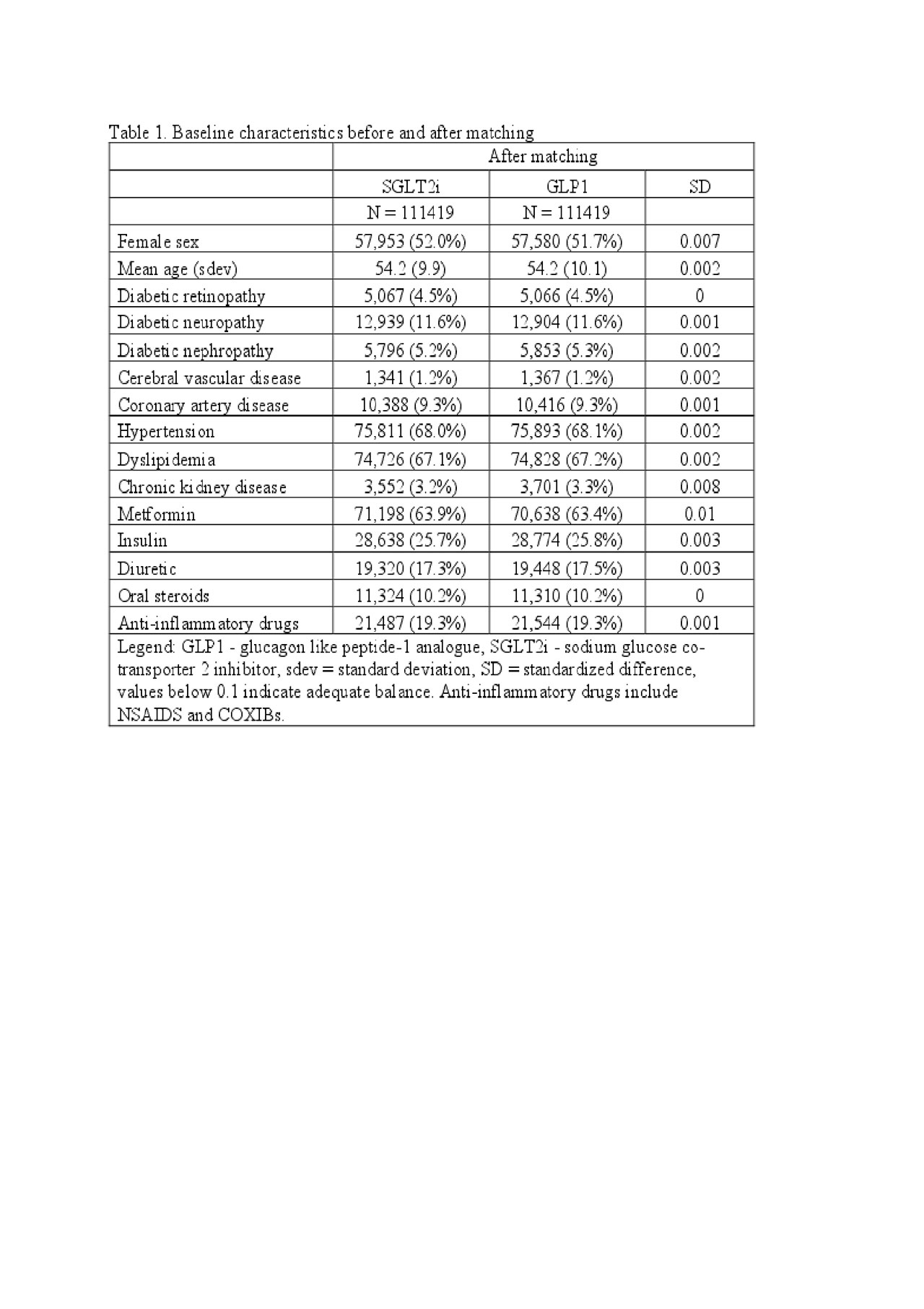
Results: We identified 295,907 adults with type 2 diabetes mellitus who were newly prescribed an SGLT2 inhibitor or a GLP1 agonist. After 1:1 propensity score matching, we identified 111,419 pairs of patients prescribed an SGLT2 inhibitor or GLP1 agonist. The mean age was 54 years, 52% were female, and 26% were prescribed insulin at baseline (Table 1). The incidence rate of gout was lower for patients prescribed an SGLT2 inhibitor (4.9 events per 1,000 person-years) compared to those prescribed a GLP1-agonist (8.1 events per 1,000 person-years), with a HR of 0.61 (95%CI 0.54-0.69) over a median follow-up of 177 days. As anticipated, SGLT2 inhibitor initiators had a lower rate of heart failure (HR 0.63, 95%CI 0.51-0.78) and a similar rate of cellulitis (HR 1.05, 95%CI 1.00-1.11) compared to patients prescribed a GLP1 agonist.
Conclusion: Adults with type 2 diabetes prescribed an SGLT2 inhibitor had a lower rate of gout compared to those prescribed a GLP1 agonist. SGLT2 inhibitors may reduce the risk of developing gout among adults with type 2 diabetes mellitus, though future studies are necessary to confirm this observation.
To cite this abstract in AMA style:
Fralick M, Chen S, Patorno E, Kim S. Assessing the Risk of Gout with Sodium Glucose Co-Transporter-2 Inhibitors: A Population-Based Cohort Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/assessing-the-risk-of-gout-with-sodium-glucose-co-transporter-2-inhibitors-a-population-based-cohort-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-the-risk-of-gout-with-sodium-glucose-co-transporter-2-inhibitors-a-population-based-cohort-study/