Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) has many symptoms, including cognitive impairment (CI), which negatively impacts social role participation and quality of life. CI is highly prevalent in SLE patients. There is a lack of data on subjective SLE-related cognitive symptoms. The Perceived Deficits Questionnaire (PDQ-20) is a patient-reported screening tool for CI in multiple sclerosis. Minimal research exists on its use in SLE. The objectives of the study are:
- To describe subjective CI in SLE across different Canadian centres using the PDQ-20 and to determine which domains are most affected.
- To study the concurrent construct validity of PDQ-20 compared to Symptom Severity Score (SS 2a), a scale used to assess cognitive symptoms, fatigue and waking unrefreshed.
Methods: SLE patients (n=398) were recruited from 7 Canadian medical centers (6 academic, 1 community). Patients completed the PDQ-20, a tool with 20 items assessing perceived cognitive difficulties on a 5-point Likert scale (0-never to 4-almost always experiencing difficulty). All 3 domains of the SS 2a were analyzed alongside PDQ-20.
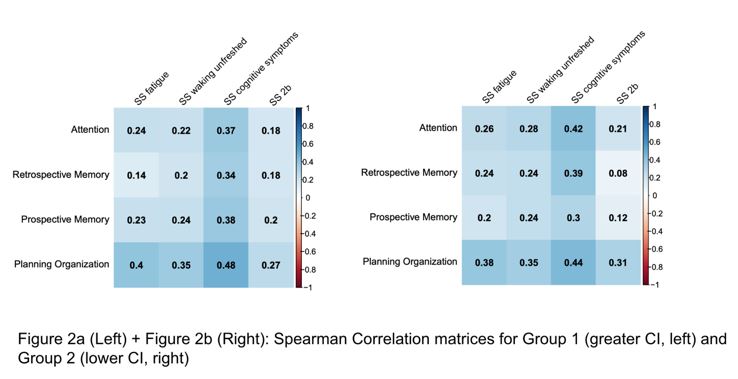
Cluster analysis was used to group patients by the severity of subjective CI using the PDQ-20. We examined the concurrent construct validity of the PDQ-20 against the SS 2a in these clusters. Spearman correlation coefficients were calculated for PDQ-20 subscales and SS 2a domains. We hypothesized that the waking unrefreshed and fatigue domains of SS 2a would show a weak-moderate correlation with all PDQ-20 subscales, while the cognitive symptoms domain of SS 2a would show a moderate-strong correlation. We also studied the known-group validity, and expected worse scores in SS 2a domains for the group with worse subjective CI.
Results: Out of 398 participants, 91.7% were female, with a mean enrollment age of 46.9 ± 13.7 years and mean SLE duration of 15.8 ± 11.8 years. The cohort had a mean SLEDAI-2k score of 3.3 ± 3.7 and mean SDI score of 1.1 ± 1.6. Additionally, 79% used antimalarial drugs, 61% immunosuppressants, 11% biologics, and 37% glucocorticoids (mean dose 7.2 ± 5.9 mg).
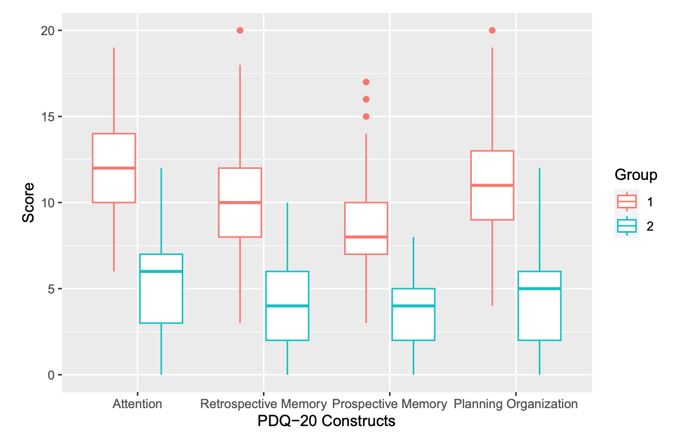
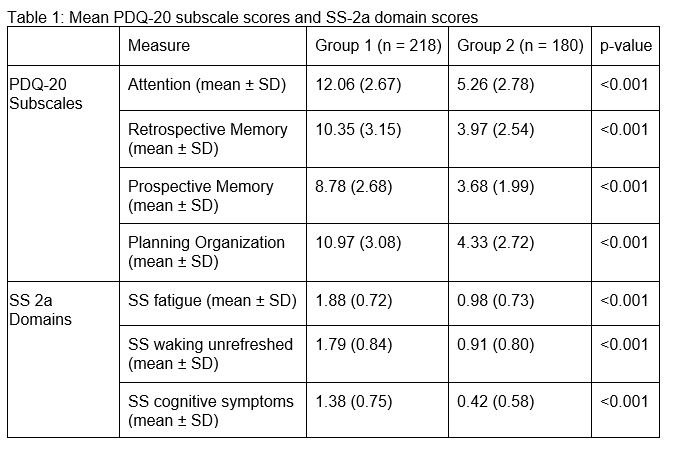
Two groups emerged: Group 1 (n=218) showed significantly higher PDQ-20 scores across all subscales compared to Group 2 (n=180), reflecting worse subjective CI. Both groups exhibited the most impairment in Attention and Planning/Organization, and the least in Prospective Memory (Figure 1). Subjective CI assessed by PDQ-20 was weakly associated with fatigue and waking unrefreshed, but moderately with cognitive symptoms in both groups (Figure 2a and 2b). Group 1 also had significantly higher mean SS 2a scores across all domains compared to Group 2 (Table 1). Both groups showed similar SLEDAI-2K (Group 1: 3.43, Group 2: 3.23) and SDI scores (Group 1: 1.08, Group 2: 1.06).
Conclusion: We identified two patient groups with different levels of subjective CI using the PDQ-20. Group 1 exhibited worse subjective CI than Group 2, with the most significant impairments in the Attention and Planning/Organization subscales of the PDQ-20. The PDQ-20 demonstrated construct validity compared to the SS 2a and may be a useful tool for measuring subjective CI among SLE patients.
To cite this abstract in AMA style:
Marzouk O, Avina-Zubieta J, Fox M, Shaw W, Ho M, Li Q, Ivory C, Fortin P, Bingham K, Keeling S, Reynolds J, Haaland D, Pope J, Lim L, Katz P, Urowitz M, Whittall Garcia L, Gladman D, Nowrouzi-Kia B, Touma Z. Assessing Subjective Cognitive Impairment in a Cohort of Canadian Systemic Lupus Erythematosus Patients: Construct Validity of PDQ-20 [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/assessing-subjective-cognitive-impairment-in-a-cohort-of-canadian-systemic-lupus-erythematosus-patients-construct-validity-of-pdq-20/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-subjective-cognitive-impairment-in-a-cohort-of-canadian-systemic-lupus-erythematosus-patients-construct-validity-of-pdq-20/