Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: This study aims to evaluate the risk of major cardiovascular events (MACE) associated with common rheumatology medications.
Methods: We conducted a retrospective pharmacovigilance analysis using the FDA Adverse Event Reporting System (FAERS) database from January 2012 to March 2024 (all the considered drugs were FDA approved in 2012). The focus was on the association of MACE—myocardial infarction (MI), stroke, angina, and cardiac failure (CF)—with the use of prednisone (PRED), methotrexate (MTX), abatacept (ABT), tofacitinib (TOF), TNF-α inhibitors [infliximab (IFX), adalimumab (ADA), etanercept (ETN), golimumab (GOL), and certolizumab (CTZ)], and rituximab (RTX) across various conditions. We excluded individuals under 18 and performed a disproportionality analysis using the reporting odds ratio (ROR).
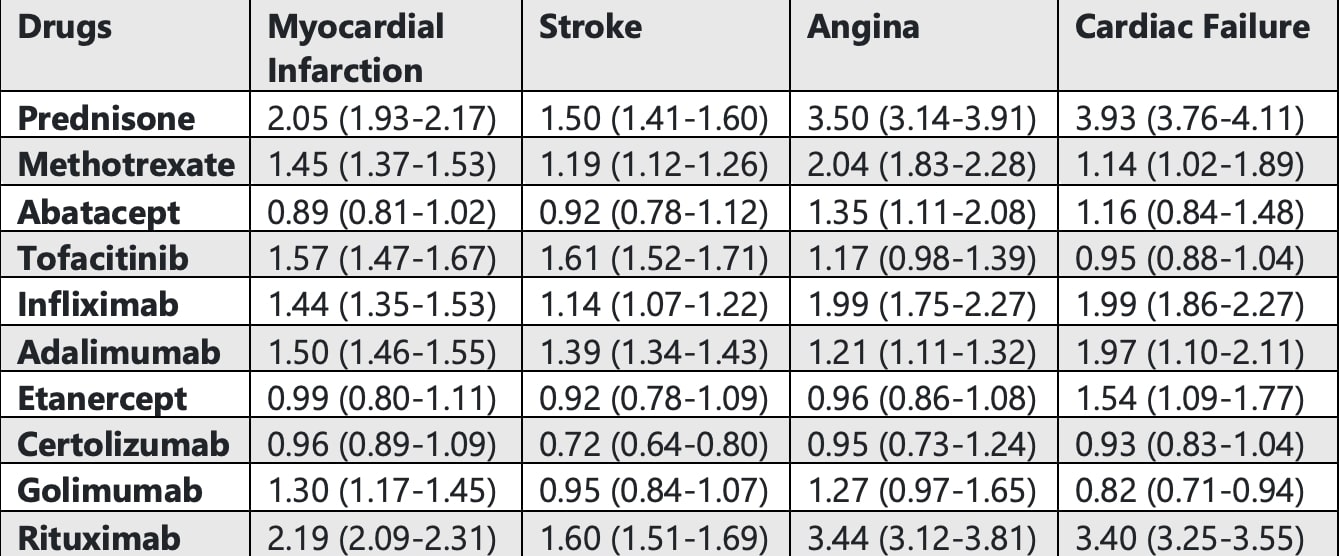
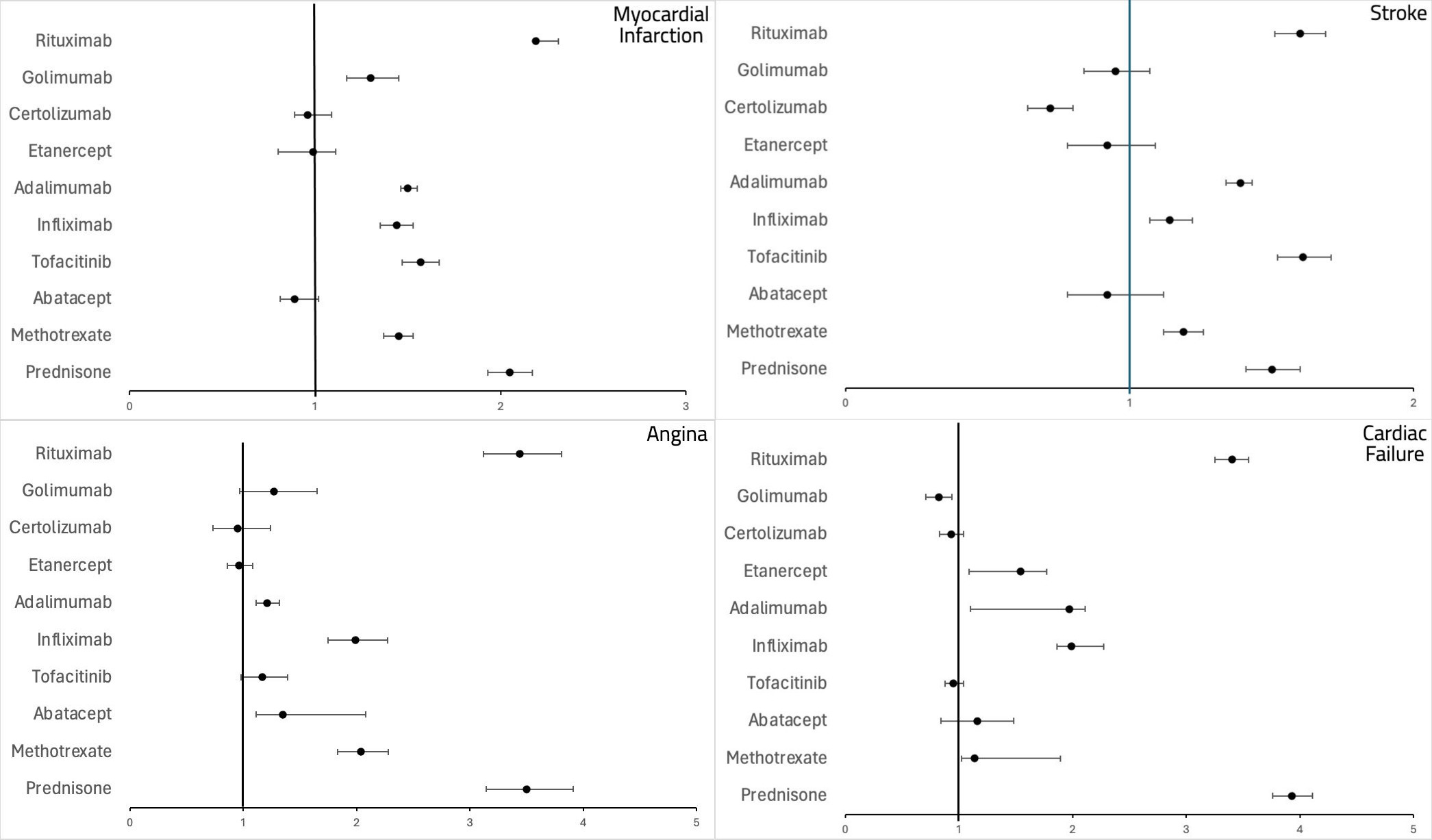
Results: The FAERS database recorded over 28 million adverse events. Stroke and CF were mainly observed in the 65-85 age group across most drugs, while MI and angina were more frequent in the 18-64 age group, except for PRED, which showed higher MI cases in the 65-85 age group. The average death rate for MI was 16% across all drugs, except for RTX, which had a rate of 31%. In CF patients, RTX and IFX had the highest death rates at 30%, followed by PRED at 24%. Women were generally more affected than men, except in MI and CF cases with RTX, where more men were affected. MI was most strongly associated with RTX use (ROR: 2.19, 95% CI: 2.09-2.31, P < 0.0001), followed by PRED (ROR: 2.05, 95% CI: 1.93-2.1, P < 0.0001). ABT, ETN, and CTZ showed a negative association with MI, although this was not clinically significant. Similarly, RTX and PRED were strongly associated with an increased risk of stroke, while CTZ showed a negative association (ROR < 1) along with ABT, ETN, and GOL, although these were not clinically significant. For angina, PRED showed the highest association, followed by RTX and MTX, with only ETN and CTZ showing a negative association that was not clinically significant. PRED and RTX were most strongly associated with congestive CF, followed by IFX, ADA, and ETN.
Conclusion: The cardiac side effects of rheumatology drugs need to be better understood. Studies have shown that patients with newly diagnosed RA have a lower MACE risk with GOL, ABT, and MTX1; MTX reduces CF-related hospitalizations2; PRED increases MACE, MI, and CF risks3; and TOF has a lower MACE risk than TNF-α inhibitors4. Our findings partially align with these studies but highlight significant unknowns about most drugs. Primary prevention through regular exercise, healthy diet, managing comorbidities like hypertension and diabetes, lipid screening, smoking cessation, and using the lowest effective dose, especially for high ROR drugs, may mitigate MACE. We recommend considering a patient’s cardiac and family history of adverse cardiac events before initiating these medications and discontinuing their use if any adverse cardiac symptoms develop. The study’s limitations include reporting bias, confounding variables, and its observational nature, which precludes establishing causality. Further controlled studies, such as randomized clinical trials or prospective cohort studies, are necessary to confirm these findings and establish causality.
To cite this abstract in AMA style:
Sondhi M, Hayat S, Muzaffar K, Umer S. Assessing Major Adverse Cardiovascular Events (MACE) Risk in Common Rheumatology Treatments [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/assessing-major-adverse-cardiovascular-events-mace-risk-in-common-rheumatology-treatments/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-major-adverse-cardiovascular-events-mace-risk-in-common-rheumatology-treatments/