Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: PsA
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Individualized treatment strategies are of high importance in the treatment of patients with active psoriatic arthritis (PsA), a heterogeneous immune-mediated disease. IL-17 inhibition has demonstrated efficacy in all domains of PsA. Nevertheless, approx. 30% of patients will not achieve remission after initiation of therapy. Therefore, the identification of patient characteristics with a high impact on treatment response is important to guide treatment choice and its adjustments in clinical routine care to promote an improved outcome. An adapted machine learning approach (cluster analysis) was used to analyze baseline (BL) patient characteristics to detect clinical patterns of disease activity in PsA patients treated in a non-interventional trial with secukinumab (SEC).
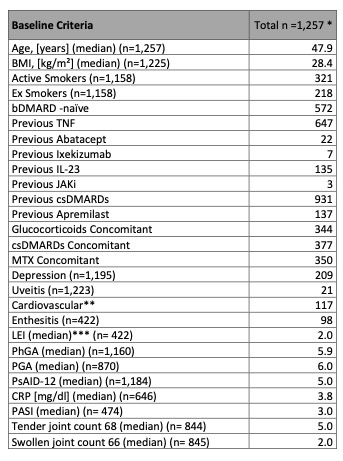
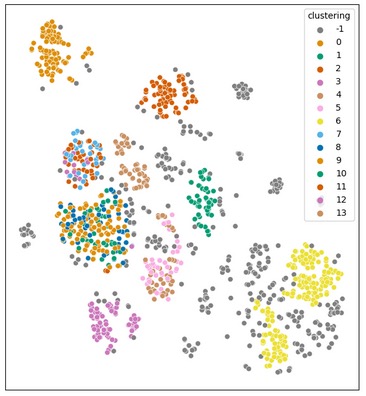
Methods: Data from 1257 patients from the German non-interventional study AQUILA with active PsA whose first SEC treatment occurred no more than 4 weeks prior to BL was analyzed. All patients were included irrespective of treatment response. We identified patient groups by applying an extended version of the machine learning method of hierarchical density-based clustering to the BL data, where features included patient and disease characteristics variables as well as standardized patient-reported outcomes (Table 1). Feature groups with high numbers of missing values were initially excluded, and subsequent clustering was performed to achieve stepwise integration of all features. At each step, an additional feature group was included in a re-clustering using a complete subset of subjects. This led to a further split of some of the original clusters. For every final cluster, disease activity over the course of the study was visualized including patient and physician-derived assessments.
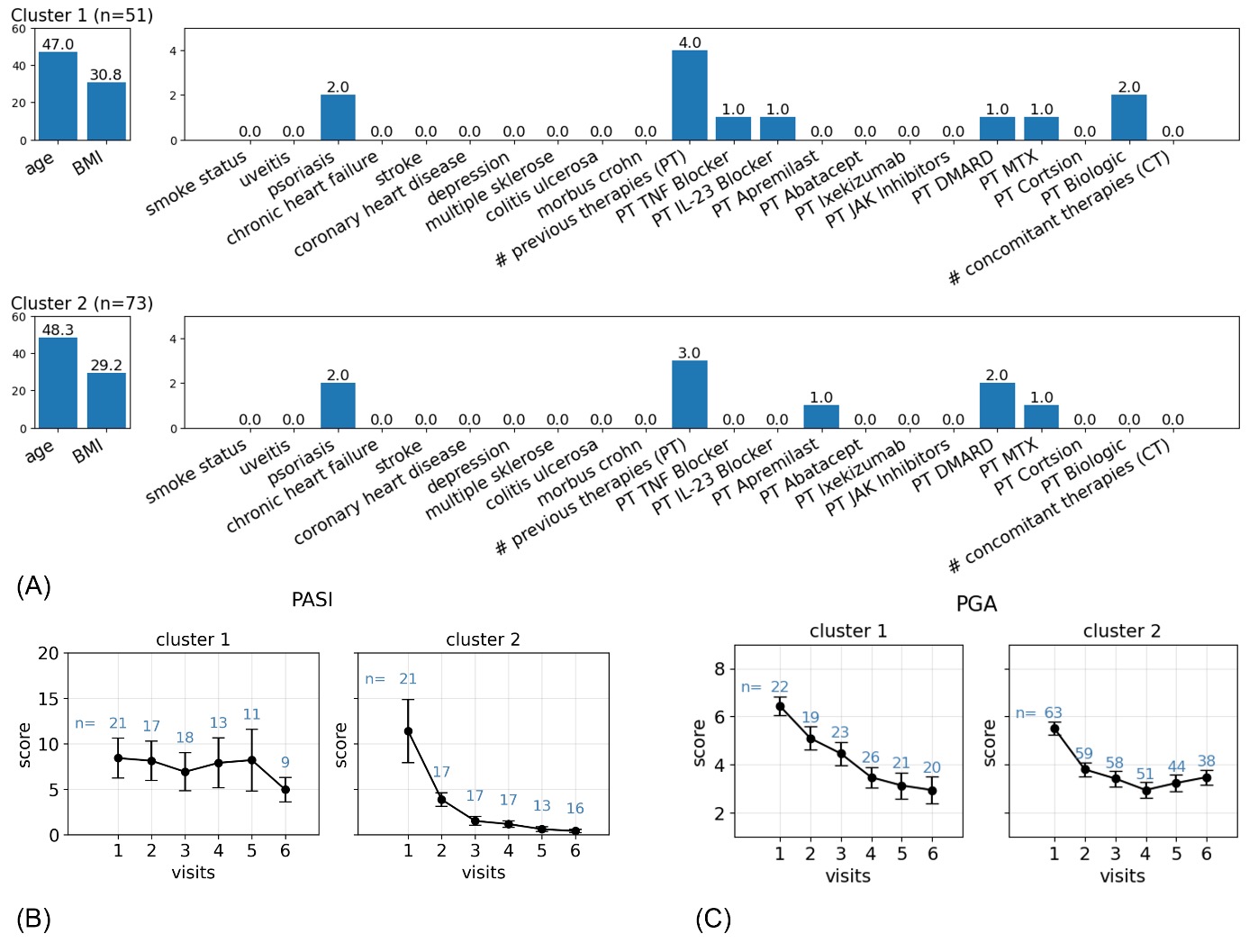
Results: The machine learning approach resulted in the categorization of 14 different clusters of patients and outliers (n=391, cluster -1) (Fig. 1). The focus is directed towards two medically relevant clusters that differ primarily in their pretreatments prior to SEC. Cluster 1 included PsA patients with predominantly bDMARD pretreatment incl. IL-23-inhibitors, while cluster 2 primarily received csDMARD pretreatments including apremilast (Fig. 2). Cluster 1, showed a greater reduction in disease activity than cluster 2 as measured by PASI and PGA (mean reduction: PASI: cluster 1: 8.0, cluster 2: 5.6; PGA: cluster 1: 2.8, cluster 2: 2.4) by week 52 (Fig. 2). However, these comparisons did not reach statistical significance for this sample.
Conclusion: We present a machine-learning approach to exploring the association between BL features of patients treated with SEC and their disease activity scores in the large PsA cohort of the AQUILA trial. The cluster analysis revealed that pretreatment with bDMARDs did not affect the efficacy of SEC compared to the predominantly csDMARD patient cohort (predominantly bDMARD naïve). PASI and PGA tend to have a stronger improvement in patients pretreated with bDMARDs than in patients pretreated with csDMARDs including apremilast after 52 weeks of treatment with SEC.
To cite this abstract in AMA style:
Koehm M, Klippstein M, Kugler S, Mackay S, Schulz D, Vodencarevic A, Wendt G, Peterlik D, Kiltz U, Brandt-Juergens J, Behrens F. Assessing Differential Effects of DMARDs on Psoriasis Area and Severity Index and Patient Global Assessment in Subgroups of Patients with Active PsA Treated over 52-weeks with Secukinumab in a Non-Interventional Trial (AQUILA) Using a Multistage Clustering Approach [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/assessing-differential-effects-of-dmards-on-psoriasis-area-and-severity-index-and-patient-global-assessment-in-subgroups-of-patients-with-active-psa-treated-over-52-weeks-with-secukinumab-in-a-non-int/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-differential-effects-of-dmards-on-psoriasis-area-and-severity-index-and-patient-global-assessment-in-subgroups-of-patients-with-active-psa-treated-over-52-weeks-with-secukinumab-in-a-non-int/