Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: : Methotrexate (MTX) Intolerance continues to occur in 20-40% patients despite low-dose folic acid (5-10mg per week) supplementation. Would a higher dose of folic acid (15-35 mg per week) be better? Two RCTs did not find any benefit (Sarah Morgan 1994, Dhir 2015). However, both included MTX naïve patients (starting methotrexate). No RCT has evaluated the impact of giving additional folic acid in those with intolerance. We have developed and validated a Methotrexate intolerance and severity assessment in Adult (MISA) score for MTX intolerance (Vijaykumar 2021), and we used it in this RCT to assess whether there is improvement by increasing dose of folic acid (+10 mg/week) in patients of methotrexate intolerance already on low-dose folic acid (5-10 mg per week).
Methods: This was a double-blind placebo-controlled trial which included patients of rheumatoid arthritis, between 18 to 75 years of age, receiving methotrexate > 3 months with methotrexate intolerance (MISA score >4) on low-dose folic acid supplementation (5-10 mg/week). They were randomized to receive either additional folic acid supplementation of 10mg/week (higher-dose folic acid) or placebo (continue low-dose folic acid). Primary outcome was change in MISA score at 12 weeks, secondary outcomes were change in serum folate and change in DAS28(3) at 12 weeks. Trial Registration CTRI/2023/04/051346
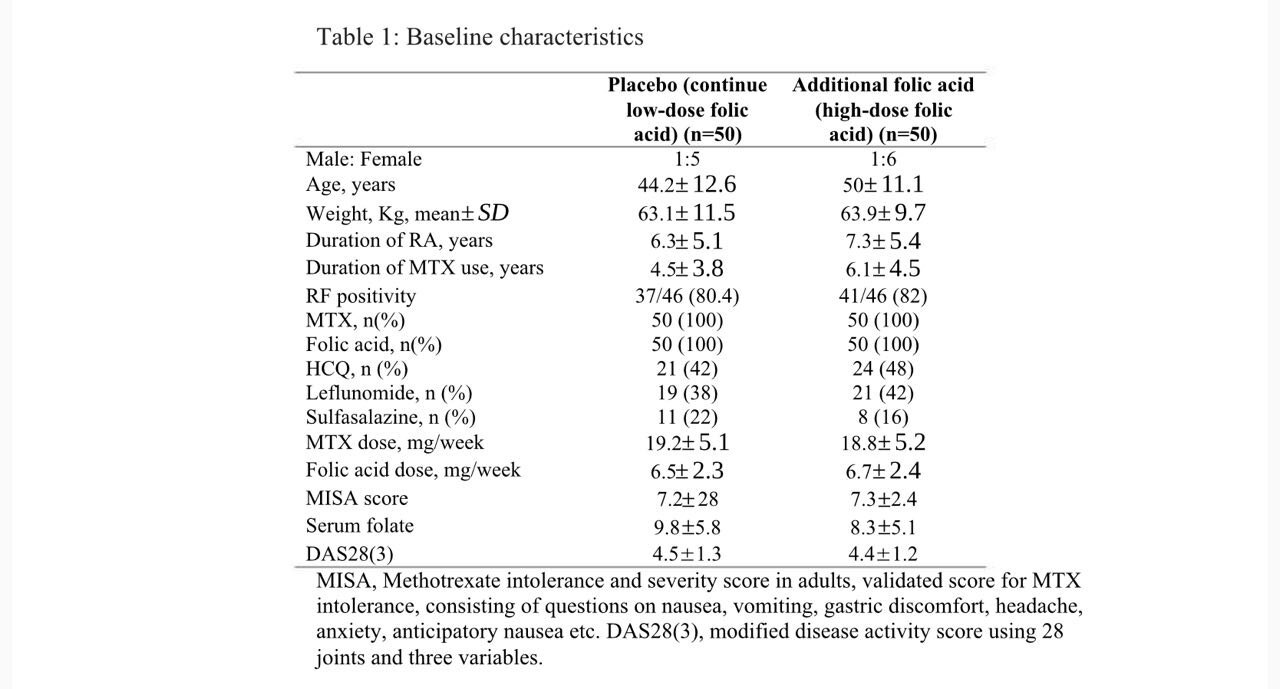
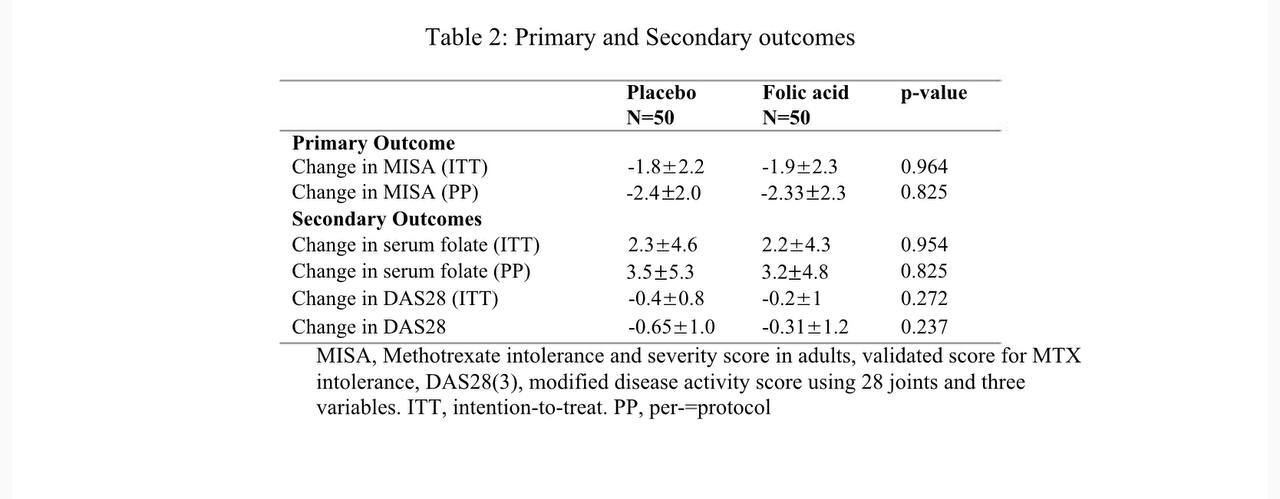
Results: A total of 100 patients were enrolled, with 50 patients randomized to placebo (continue low-dose folic acid) and additional folic acid (higher-dose folic acid) group. Mean age was 44.2 and 50 years and MTX dose was 19.2 and 18.8 mg per week respectively. There was a drop-out in 20 patients, 4 patients due to adverse effects and 16 lost to follow up. The primary outcome, change in MISA score in both groups was not significantly different between the two groups (Table 2, Figure 1). We could do the serum folate levels and DAS28(3) in only 68 and 63 patients, both of which did not show any significant difference between the groups. (Table 2)
Conclusion: There was no significant improvement in MTX intolerance with additional supplementation of 10 mg of folic acid per week to patients with methotrexate intolerance on low-dose folic acid supplementation.
To cite this abstract in AMA style:
Dhir V, Koneti H, Sharma A, jain s, naidu s. Assess the Effect of Increasing the Dose of Folic Acid Supplementation in Patients of Rheumatoid Arthritis with Methotrexate Intolerance – A Randomised Controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/assess-the-effect-of-increasing-the-dose-of-folic-acid-supplementation-in-patients-of-rheumatoid-arthritis-with-methotrexate-intolerance-a-randomised-controlled-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assess-the-effect-of-increasing-the-dose-of-folic-acid-supplementation-in-patients-of-rheumatoid-arthritis-with-methotrexate-intolerance-a-randomised-controlled-trial/