Session Information
Date: Monday, November 11, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Treatments, Outcomes, & Measures
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose:
To compare efficacy and safety of biological disease-modifying antirheumatic drugs (bDMARDs) between elderly-onset rheumatoid arthritis (EORA) and young-onset rheumatoid arthritis (YORA) patients.
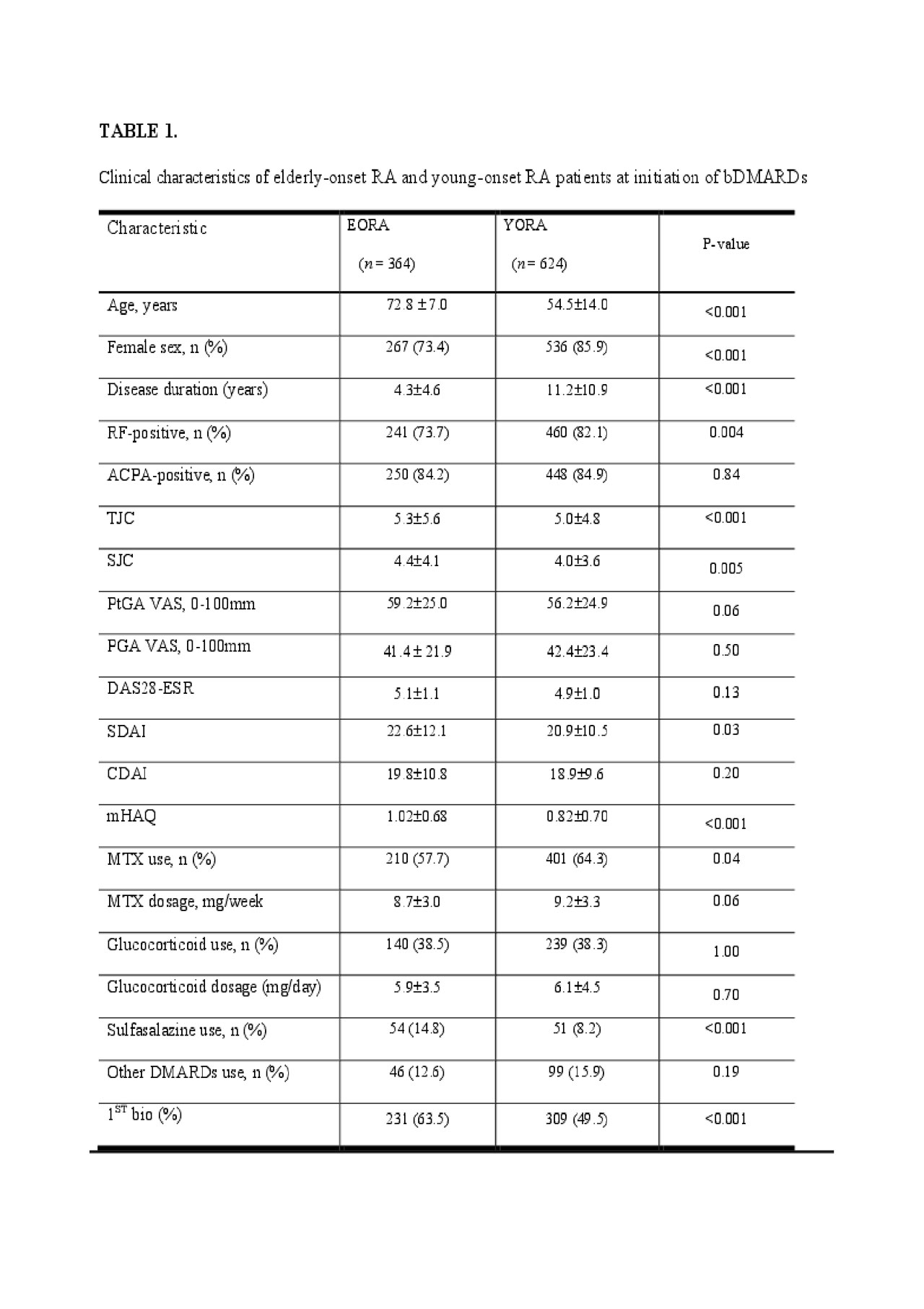
Methods: Patients with rheumatoid arthritis (RA) aged ≧18 years enrolled in a Japanese multicenter observational registry between Sep 2009 and December 2017 who had ≧3.2 disease activity score in 28 joints-erythrocyte sedimentation rate (DAS28-ESR) when initiating bDMARDs were included. EORA was defined as RA with onset at 60 or over. Considering selection bias in the estimation of effects of time-varying treatments and clustering effects by individual, a generalized estimating equation model with an inverse probability of treatment weighting was used to assess the relationship between age onset and clinical effectiveness at 48 weeks. Primary outcome was Clinical Disease Activity Index (CDAI) score at 48 weeks. Secondary outcomes included biologic retention at 48 weeks, achievement of CDAI remission, and low disease activity (LDA)/remission. Biologic retention rate was compared using a cox proportional hazards model.
Results: Among a total of 7183 patients in the registry, proportion of patients on bDMARDs was lower in the EORA as compared to the YORA (18.3 % vs 28.0 %, p < 0.001). Of the 989 bDMARDs initiators, 364 (36.8%) were identified as EORA. After adjusting for differences in baseline characteristics between the two age groups, there was no significant difference in CDAI score at 48 weeks (1.01, 95% CI=-0.62-2.64, p=0.22). There was a trend of lower remission in the EORA (OR=0.52, 95%CI=0.24-1.14, p=0.10), but LDA/remission rate was similar (OR=0.86, 95%CI=0.29-2.52, p=0.77). Drug maintenance rates (HR=0.95, 95%CI=0.55-1.35, p=0.78) and adverse events discontinuation rates (HR=0.78, 95%CI=0.38-1.18, p=0.22) were similar between the two age groups adjusting other confounders.
Conclusion: In RA patients initiating bDMARDs, improvements in clinical disease at 48 weeks were comparable between EORA and YORA. Drug maintenance and adverse events discontinuation rates were similar between the two age groups.
To cite this abstract in AMA style:
Jinno S, Onishi A, Akashi K, Hashimoto M, Yamamoto W, Murata K, Takeuchi T, Kotani T, Maeda Y, Ebina K, Son Y, Amuro H, Hara R, Katayama M, Saegusa J, Morinobu A. Are There Differences in Efficacy and Safety of Biological Disease-modifying Antirheumatic Drugs Between Elderly-onset and Young-onset Rheumatoid Arthritis? [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/are-there-differences-in-efficacy-and-safety-of-biological-disease-modifying-antirheumatic-drugs-between-elderly-onset-and-young-onset-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/are-there-differences-in-efficacy-and-safety-of-biological-disease-modifying-antirheumatic-drugs-between-elderly-onset-and-young-onset-rheumatoid-arthritis/