Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Ensuring study participants are representative of the general population is important to ensure that efficacy and safety findings of clinical trials are generalizable in clinical practice. The aim of this study was to analyse if phase 3 clinical trials of gout medications approved by the US Food and Drug Administration (FDA) included participants representative of the US general population with gout.
Methods: Data from phase 3 clinical trials of gout medications approved by the FDA from 2009 to 2023 were analysed. Data were extracted for the demographic variables of sex, age and ethnicity. Comorbidity data were extracted for hypertension, myocardial infarction, heart failure, nephrolithiasis, chronic kidney disease, body mass index (BMI) ≥ 30 kg/m2 and diabetes. In trials in which only mean and standard deviation were provided, the variable was assumed to be normally distributed. Data from the trials were compared with published data from participants with gout in the 2007 – 2008 and 2015 – 2016 US National Health and Nutrition Examination Survey (NHANES) (1, 2). Data were pooled using a random effects model and presented as a percentage and 95% confidence interval.
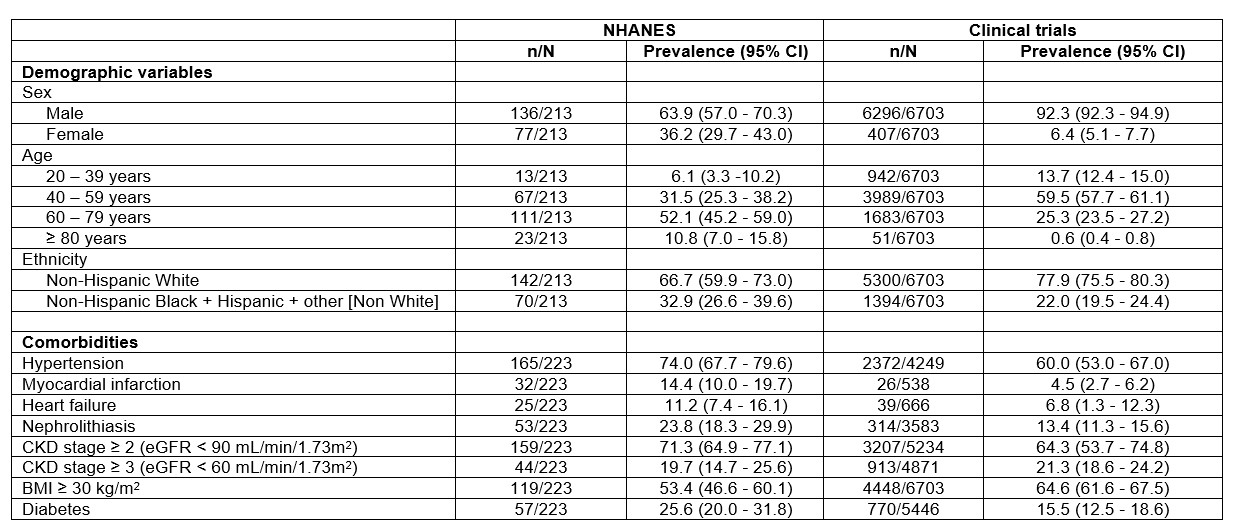
Results: Twelve phase 3 clinical trials were included. The approved gout medications were febuxostat, colchicine, pegloticase, lesinurad, and canakinumab. Reported data from NHANES and pooled data from all analysed trials are shown in the Table. Compared with the US NHANES gout population, there was over-representation of men, younger people, and people of non-Hispanic White ethnicity in the clinical trials. People with hypertension, prior myocardial infarction, nephrolithiasis, and diabetes were under-represented, and people with BMI ≥ 30 kg/m2 were over-represented in the clinical trials.
Conclusion: Clinical trials of gout medications approved by the FDA since 2009 have not enrolled a study population that is representative of the US population with gout, based on demographic features and cardiometabolic comorbidities. For broader applicability to the general population with gout, future phase 3 trials should ensure representative inclusion of women, older individuals, diverse ethnicities, and those with comorbid health conditions that are commonly experienced by people with gout.
References:
1. Zhu, Am J Med, 2012, 25(7):679-687
2. Chen‐Xu, Arthritis Rheumatol, 2019;71(6):991–9
To cite this abstract in AMA style:
Liu J, Gamble G, Dalbeth N. Are Participants in Gout Clinical Trials Representative of People with Gout in the General Population? [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/are-participants-in-gout-clinical-trials-representative-of-people-with-gout-in-the-general-population/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/are-participants-in-gout-clinical-trials-representative-of-people-with-gout-in-the-general-population/