Session Information
Date: Tuesday, November 9, 2021
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II (1565–1583)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: AR882, a novel uric acid transporter 1 (URAT1) inhibitor is being developed for the treatment of gout with hyperuricemia. In Phase 1 and Phase 2 studies, AR882 exhibited long lasting serum urate (sUA) lowering effect and good safety in healthy subjects and patients with gout. In a Phase 1 open-label, single-dose study with oral administration of [14C] AR882, the absorption, metabolism, and excretion (AME) profile of AR882 was investigated in healthy subjects.
Methods: Six (6) healthy male subjects received a single oral dose of 100 mg (250 μCi) [14C]AR882 under fasting conditions. Serial blood, urine, and fecal samples were collected up to 144 hours postdose, and then at 24-hour intervals thereafter until the subject was discharged (up to 288 hours). Total radioactivity in plasma, whole blood, urine, and fecal samples was measured using liquid scintillation counting methods. Concentration of AR882 and active metabolite, AR896, in plasma and urine was determined using validated liquid chromatography with tandem mass spectrometry (LC-MS/MS) methods. Metabolite profiling in each matrix and structure identification were performed using high resolution accurate mass LC-RAD-MS/MS with in-line fraction collection and off-line counting to obtain [14C]- radiochromatographic profiles and provide information on the nature of the radioactive components present. In addition, the metabolite profile of AR882 comparison were made cross species.
Results: All 6 subjects completed the full study except one subject withdrew after 7 days due to personal reason. The overall total recovery was 89.2% (34% in urine and 55% in feces) of [14C]AR882 administered and recovery was complete 10 days after dosing. Within 24 hours approximately 20% and 8% of dose was recovered in urine and feces, respectively. Both AR882 and total radioactivity were readily absorbed with terminal half-life of approximate 20 hours. AR882 was accounted for 88% of the circulating radioactivity followed by its active metabolite AR896 (8%). The renal clearance was approximately 0.02 mL/min and 3.20 mL/min for AR882 and AR896, respectively. AR896 exhibited weak URAT1 inhibition and was identified to contribute to the sustained inhibition of URAT1 and sUA lowering effect of AR882 in a parent-metabolite pharmacokinetics/pharmacodynamic model (Figure). Metabolic pathway of AR882 included oxidation, sulfation, glucuronidation, and hydrolysis. There were no disproportional or unique human metabolites identified when compared to AME profiles in rats and monkeys. Based on the metabolic profile in feces and recovery of radioactivity in urine, at least 80% AR882 was absorbed.
Conclusion: The sustainable sUA lowering effect of AR882 observed in clinical studies is attributed to the pharmacological effects of parent drug and its active metabolite as well as its favorable PK properties and balanced metabolic and elimination pathways.
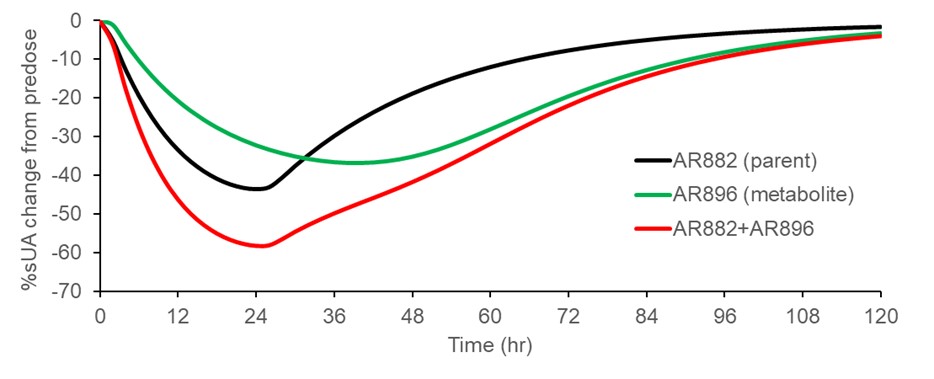 Simulated Serum Urate Lowering Effect of AR882 (Parent) and AR896 (Metabolite) following a Single Oral Dose of 100 mg AR882 based on PK/PD Model
Simulated Serum Urate Lowering Effect of AR882 (Parent) and AR896 (Metabolite) following a Single Oral Dose of 100 mg AR882 based on PK/PD Model
To cite this abstract in AMA style:
Yan R, shen z, Polvent E, hingorani v, Yan S, Yeh L. AR882, a Novel Uricosuric Agent, Exhibited Favorable Pharmacokinetic Profile and Balanced Excretion and Metabolic Pathways in a Human AME Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/ar882-a-novel-uricosuric-agent-exhibited-favorable-pharmacokinetic-profile-and-balanced-excretion-and-metabolic-pathways-in-a-human-ame-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ar882-a-novel-uricosuric-agent-exhibited-favorable-pharmacokinetic-profile-and-balanced-excretion-and-metabolic-pathways-in-a-human-ame-study/
