Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Treatment goals for long-term control of skin and joint symptoms in active psoriatic arthritis (PsA) include clinically important changes in DAS-28 (CRP), achievement of remission in DAS-28 (CRP), reduction in swollen joint count (SJC), and decrease in skin disease.1 PALACE 3 (NCT01212770) included PsA patients (pts) with active joint disease and an active skin lesion at the time of enrollment. We report the impact of apremilast (APR) on PsA manifestations over 4 years.
Methods: Pts were stratified by baseline (BL) DMARD use (yes/no) and psoriasis involvement of the body surface area (<3%/≥3%) and randomized (1:1:1) to placebo (PBO), APR 30 mg BID (APR30), or APR 20 mg BID (APR20). After the 24-week PBO-controlled phase, all pts were treated with APR30 or APR20 and could enroll in the long-term extension. Efficacy assessments were conducted through Week 208.
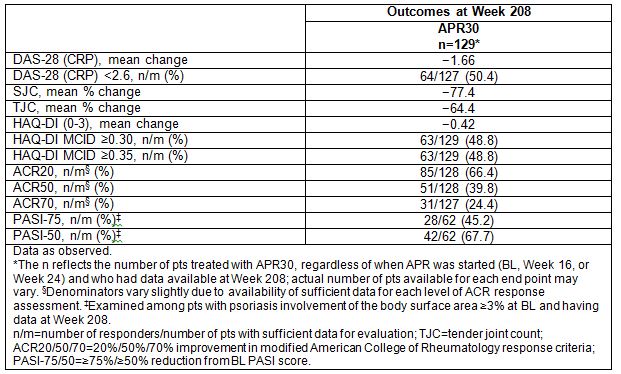
Results: 505 pts were randomized and received ≥1 dose of study medication (PBO: n=169; APR30: n=167; APR20: n=169). A total of 91% (227/249) of pts starting the fourth year of APR therapy completed the Week 208 visit. Pts treated with APR30 demonstrated sustained decreases in disease activity at Week 208, as shown by mean change from BL in DAS-28 (CRP) of −1.66; 80.3% achieved good/moderate EULAR response and 50.4% achieved DAS-28 (CRP) remission. Sustained effect on inflammation at Week 208 was also demonstrated by mean/median percent changes in SJC, a marker of inflammatory activity, of −77.4%/−100.0% (Table); 64.8% of pts had an SJC 0 or 1. Decreases in disability and maintenance of functionality were shown by sustained improvements in Health Assessment Questionnaire-Disability Index (HAQ-DI) scores (Table). A continued effect on skin disease was shown by decreases in skin involvement, as measured by the Psoriasis Area and Severity Index (PASI); 54.7% of APR30 pts had BL PASI >5 and 27.3% had BL PASI >10; at Week 208, 64.5% had PASI <3 and 77.4% had PASI ≤5. PASI-75 and PASI-50 response rates also signified clinically significant relief (Table). In pts treated with APR20, similar findings were observed at Week 208. No new safety concerns were identified through 208 weeks of APR30 therapy. During Weeks >156 to ≤208 of APR30 exposure, the only AE occurring in ≥5% of pts was nasopharyngitis; most AEs were mild or moderate in severity. Serious AEs occurred in 7.2% of APR30 pts Weeks >156 to ≤208, similar to rates in earlier study periods. Few discontinuations due to AEs (0.7%) occurred over Weeks >156 to ≤208. The APR20 safety profile was similar to that of APR30.
Conclusion: Over 208 weeks, APR demonstrated sustained and clinically important improvements in PsA signs and symptoms, including physical function and associated psoriasis, among pts continuing the study. APR was generally well tolerated with an acceptable safety profile. Reference: 1. Gossec et al. Ann Rheum Dis. 2015 Dec 7. doi: 10.1136/annrheumdis-2015-208337.
To cite this abstract in AMA style:
Edwards CJ, Blanco FJ, Crowley JJ, McIlraith M, Shah K, Delev N, Teng L, Birbara CA. Apremilast Is Associated with Long-Term DAS-28 (CRP) Remission and Improvements in Skin Disease: Results from a Phase III Study in DMARD/Biologic-Experienced Active Psoriatic Arthritis Patients [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/apremilast-is-associated-with-long-term-das-28-crp-remission-and-improvements-in-skin-disease-results-from-a-phase-iii-study-in-dmardbiologic-experienced-active-psoriatic-arthritis-patients/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/apremilast-is-associated-with-long-term-das-28-crp-remission-and-improvements-in-skin-disease-results-from-a-phase-iii-study-in-dmardbiologic-experienced-active-psoriatic-arthritis-patients/