Session Information
Date: Sunday, November 10, 2019
Title: Pediatric Rheumatology – ePoster I: Basic Science, Biomarkers, & Sclerodermic Fever
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic JIA (sJIA) and Adult Onset Still’s Disease may represent a disease continuum1 of the same autoinflammatory disorder, Still’s Disease. Current challenges in its management include the complex disease clinical phenotypes (systemic/rheumatic symptoms) and the absence of optimal treatment guidelines. Several efforts are being made to identify the best targeted strategy to take advantage of the “window of opportunity” and prevent complications and damage2. Systems biology-based methods are becoming reliable tools to understand the molecular effects of drugs in complex clinical settings. In this study, we constructed in silico models to explore and compare the mode of action (MoA) in Still’s Disease of current biologicals, anakinra (ANA), canakinumab (CAN) and tocilizumab (TCZ), in addition to non-biological drugs such as corticosteroids and methotrexate, aiming to advance towards an optimal treatment approach.
Methods: Therapeutic Performance Mapping System was used to create Still’s Disease pathophysiology and drug MoA models. Drugs’ efficacies were compared using artificial neuronal networks, and detailed MoA of IL-1β and IL-6 blockers was modeled using sampling methods. Available expression data in sJIA patients was used for model validation (GSE80060, GSE21521, GSE8361, GSE7753, GSE76492).
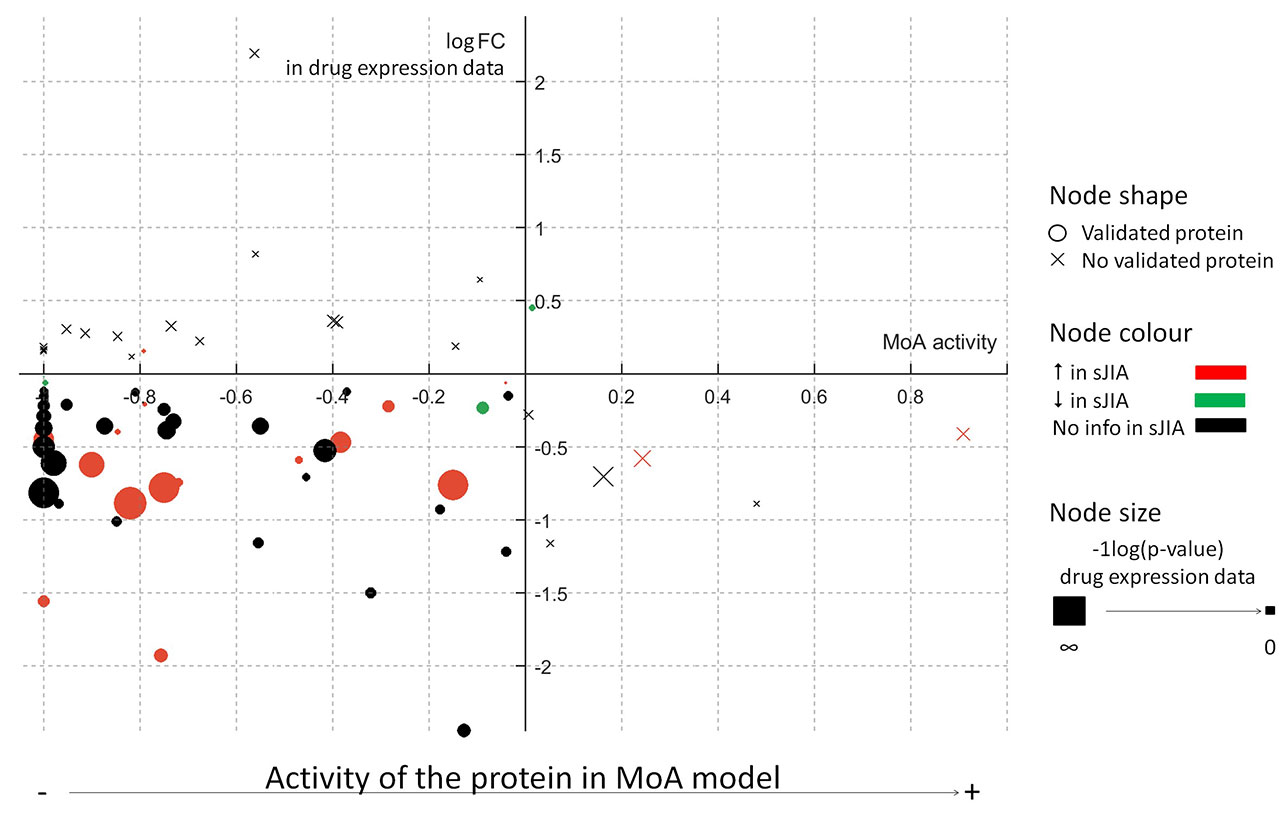
Results: The models reflected human physiology ( >90% accuracy) and Still’s Disease pathophysiology (Figure 1A). Biologicals were found more efficient than non-biologicals (Table 1). IL-1 blockers behaved similarly (CAN as IL-1β-specific blocker was used for further analyses) and presented an innate immune system-centered mechanism, while TCZ acted over the adaptive immune system. A detailed evaluation of the MoA of CAN and TCZ on the innate immune system showed some well-known proteins (Figure 1B) differentially modulated. While CAN inhibits NF-κB, CXCL8 and S100A9 more effectively, CD64 (FCGR1A) is preferentially inhibited by TCZ. The CAN and TCZ MoA models reproduced 67% of the information obtained from expression data (Figure 2).
Conclusion: The created in silico Still’s Disease models reproduce known clinical and molecular findings, which render them a good tool for future patient profiling, biomarker identification and treatment strategy designing. According to the models, the biologicals tested for proof-of-concept purposes provide a more pathophysiology-directed MoA than non-biological drugs, and are similarly effective on both systemic and rheumatic disease features. IL-1 blockers, specifically CAN, might be more efficient than TCZ in initial autoinflammatory/systemic phases of Still’s Disease that is dominated by innate immune dysregulation. Key innate immune mediators are hereby proposed to explain the differences observed between CAN and TCZ MoA. Our systems biology data may thus support the development of therapeutic strategies fostering early intervention with CAN during the window of opportunity to prevent the development of destructive chronic arthritis and treatment- or disease-associated complications long-term.
References:
- Luthi F, et al. Clin Exp Rheumatol. 2002
- Nigrovich PA, et al. Arth Rheum. 2014
To cite this abstract in AMA style:
Segu-Verges C, Coma M, Kessel C, Smeets S, Foell D, Aldea A. Application of Systems Biology-Based In Silico Tools for Optimal Treatment Strategy Identification in Still’s Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/application-of-systems-biology-based-in-silico-tools-for-optimal-treatment-strategy-identification-in-stills-disease/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/application-of-systems-biology-based-in-silico-tools-for-optimal-treatment-strategy-identification-in-stills-disease/