Session Information
Date: Sunday, November 7, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Measurements (0739–0763)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Validated patient-reported outcome (PRO) measures play important roles in assessing and managing of patients with rheumatic diseases. However, use of these validated scales may not be feasible in the setting of telehealth encounters. Likewise, completion of physician-reported measures of disease activity may not be possible via telehealth because, for example, joint examinations and global assessments cannot be conducted reliably. In both cases, acceptable proxies are needed for use in telehealth as this method of care delivery remains widely used. We examined the relationship between (1) simple, single-item ratings of pain, function, and fatigue with validated scales measuring the same constructs, and (2) patient and physician ratings of disease activity in patients with rheumatoid arthritis.
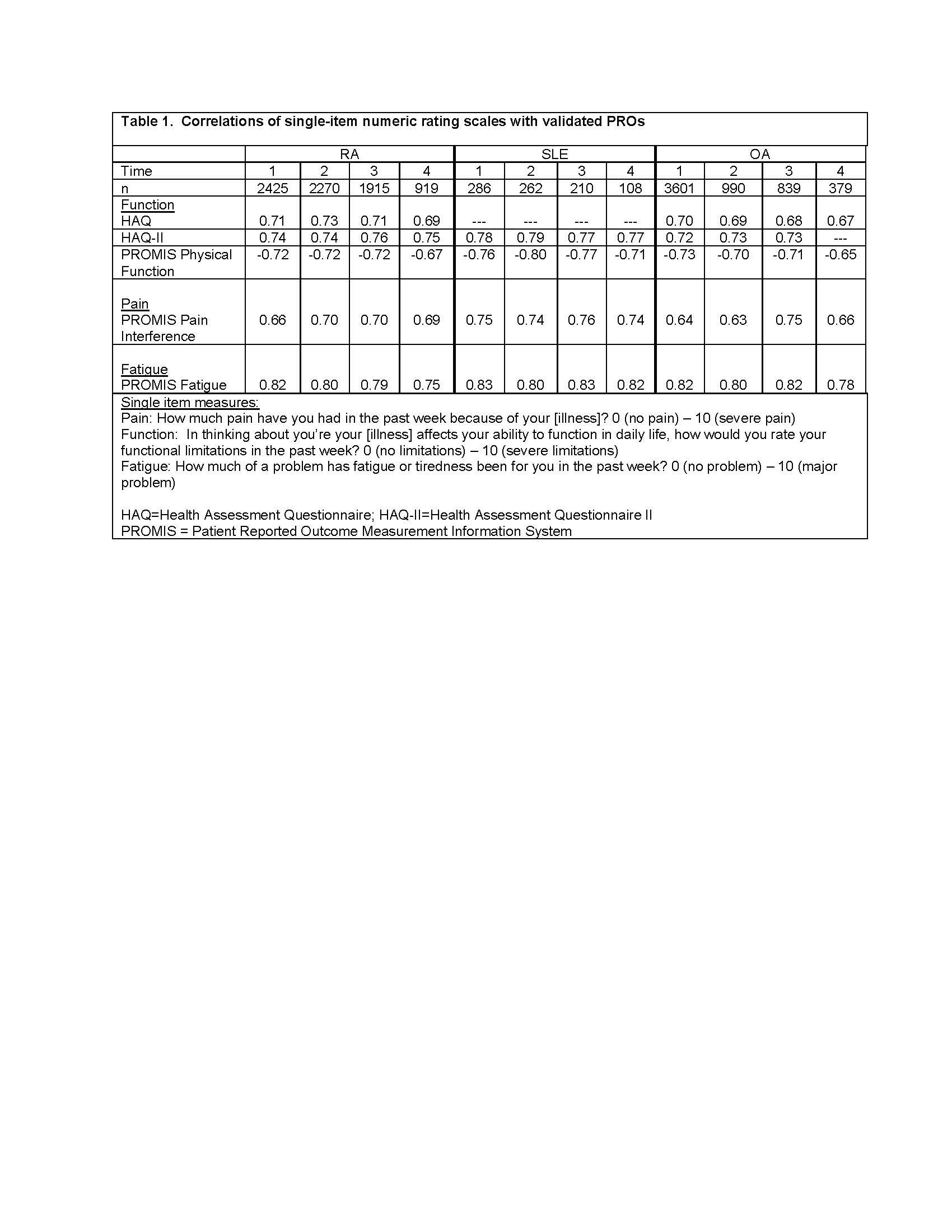
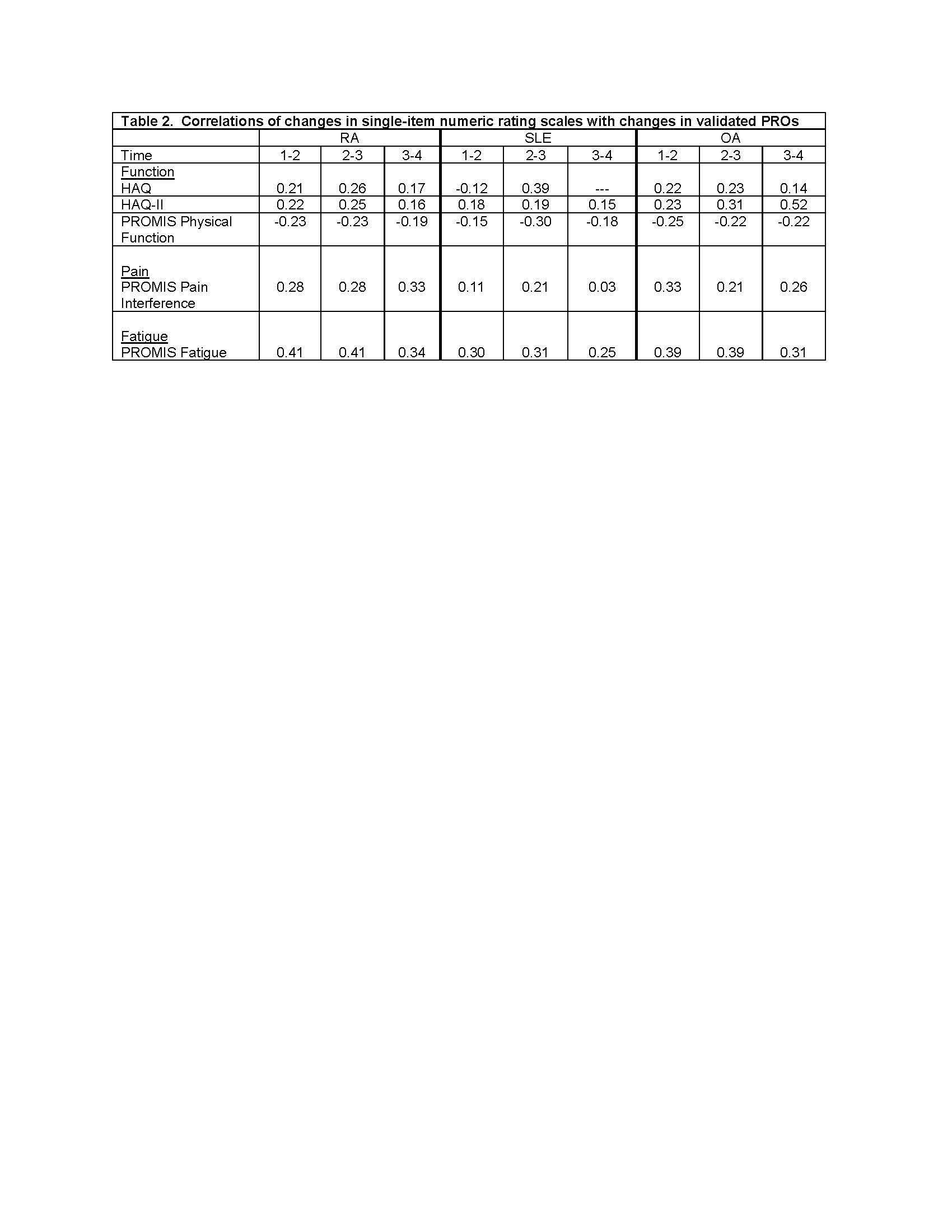
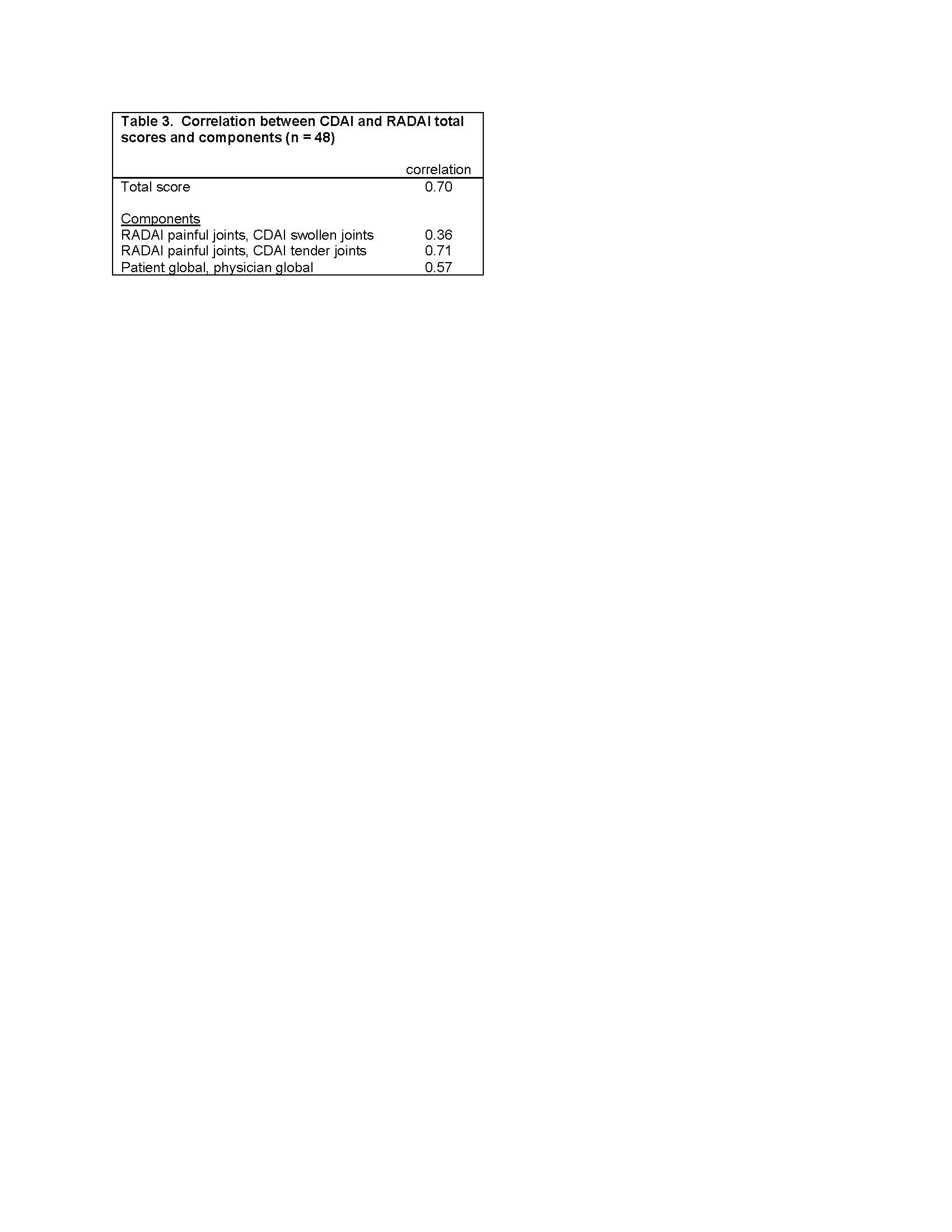
Methods: Analysis 1 uses data from FORWARD, The National Databank for Rheumatic Diseases, a longitudinal registry of individuals with rheumatic diseases. Data are regularly collected via semi-annual questionnaires. Within each questionnaire, pain, function, and fatigue are assessed by both single-items and validated scales (Table 1). Spearman correlations examine the associations between single-item and scales for each construct at 4 different questionnaire administrations and between 6-month changes in item and scale responses. Analyses were conducted separately for rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and osteoarthritis (OA). Analysis 2 uses data from a study of people with rheumatoid arthritis in which both the Clinical Disease Activity Index (CDAI) and patient-reported Rheumatoid Arthritis Disease Activity Index (RADAI) were completed. Spearman correlations examine associations between CDAI and RADAI scores and components (global assessments, joint counts).
Results: Analysis 1: Participants with RA (n=7478) had mean age 68±11 years, were 92% white and 83% female; those with SLE (n=893) had mean age 63± 13 years, were 83% white, and 94% female; and those with OA (n=3371) had mean age 70± 10 years, were 95% white, and 86% female. Cross-sectional correlations between single items and scales were uniformly medium to large (Table 1). Correlations between change scores, however, were small (Table 2)..
Analysis 2: Participants (n=48) had mean age 58 ± 12 years, were 76% female, 36% white, 7% Asian, 14% Black, and 43% other/multi-racial, and 36% LatinX ethnicity. Correlations between CDAI and RADAI total scores was 0.70, RADAI painful joint count and CDAI tender joints 0.71, and RADAI and CDAI global assessments 0.57.
Conclusion: Cross-sectional group associations between single-item and scale responses were high suggesting the potential for usefulness in telehealth settings where rheumatologists may not have sufficient time to complete longer validated measures nor data from a physical exam. Correlations in changes, however, were low, perhaps reflecting reduced precision of the single-item measures, which might limit the usefulness of these measures for following patients over time. The moderate-high associations between CDAI and RADAI scores and components suggests that remote patient-completed assessments of disease activity may be useful in telehealth settings.
To cite this abstract in AMA style:
Katz P, Subash M, Bergman M, Michaud K. Application of Existing Patient-Reported Outcome Measures for Telehealth Encounters: What’s Feasible? [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/application-of-existing-patient-reported-outcome-measures-for-telehealth-encounters-whats-feasible/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/application-of-existing-patient-reported-outcome-measures-for-telehealth-encounters-whats-feasible/