Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: APS ACTION “Registry” was created to study long-term outcomes in persistently antiphospholipid antibody (aPL)-positive patients with and without other systemic autoimmune diseases. Our primary objective was to determine whether clinically significant aPL profiles at baseline remain stable over time.
Methods: A web-based data capture system is used to store patient demographics and aPL-related medical history. Inclusion criteria are positive aPL, based on the Updated Sapporo APS Classification Criteria, tested at least twice within one year prior to enrollment. Patients are followed every 12±3 months with clinical data and blood collection. For this prospective analysis of available follow-up (f/u) aPL tests, clinically significant aPL profile was defined as positive lupus anticoagulant (LA) test and/or aCL/aβ2GPI IgG/M >40U. Stable aPL profile was defined as a clinically significant aPL profile in at least two-thirds of f/u measurements. Univariate and multivariable generalized linear mixed models with logit link were used to assess the effect of time and other variables of interest on odds of clinically significant aPL profile. Wilcoxon rank-sum and Fisher’s exact tests were employed to compare clinical characteristics of patients with stable versus unstable aPL profiles.
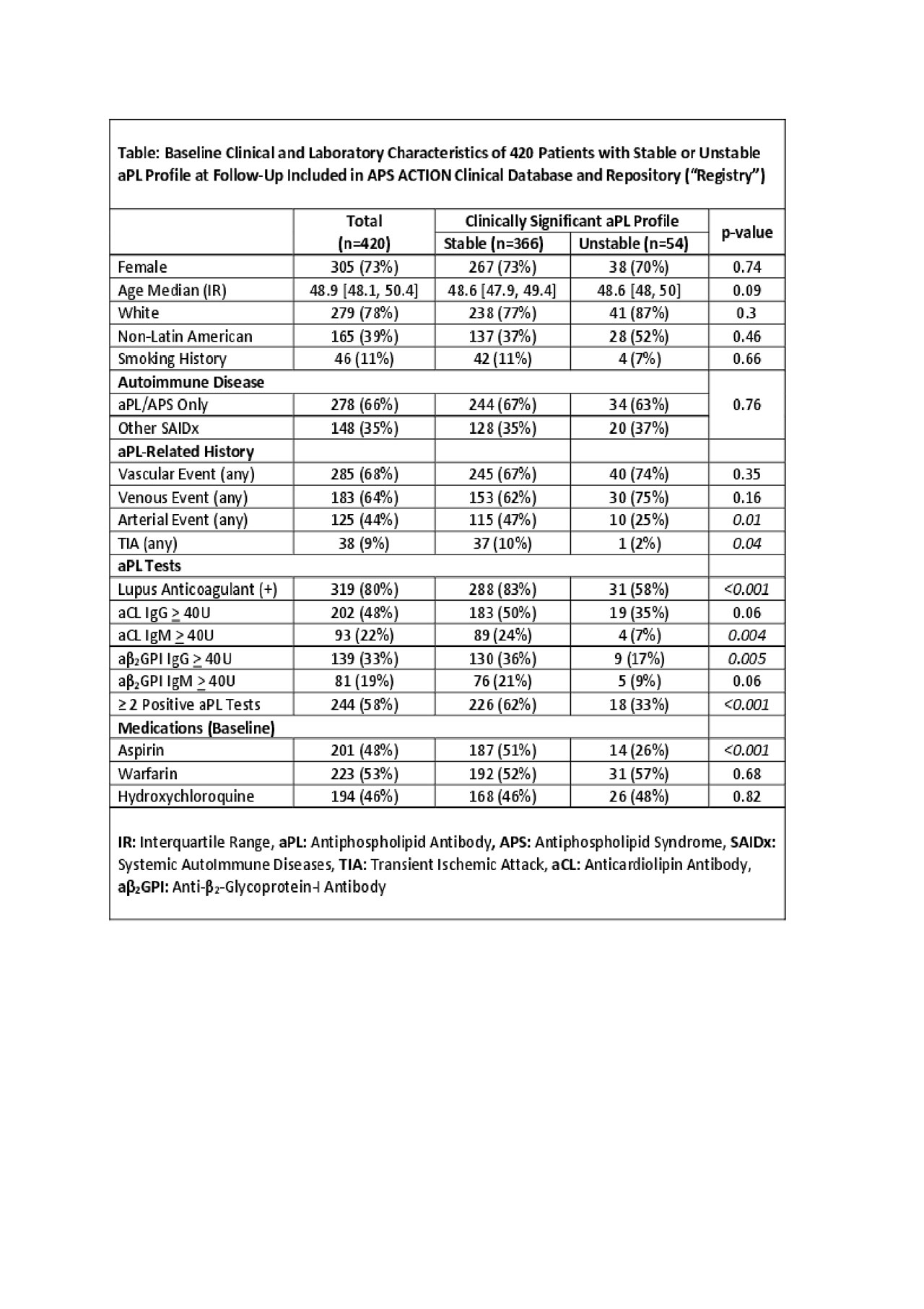
Results: As of January 2019, 796 patients were enrolled from 26 centers worldwide, 482 had f/u visits with aPL results, and 472 patients had a clinically significant aPL profile at baseline. Based on aPL profiles at f/u visits (median follow up: 5.1 years [interquartile range [IR]: 4.3, 5.8]; median number of f/u visits with aPL profiles: 2 [interquartile range: 1, 3]), 366/472 (78%) patients had stable aPL profiles over time (54 [11%] unstable; 52 [11%] inconclusive). Time did not affect odds of maintaining a clinically significant aPL profile at f/u (p=0.906). In multivariable analysis, time, age, concomitant systemic autoimmune disease (mainly lupus), smoking history, and hydroxychloroquine use did not affect odds of maintaining a clinically significant aPL profile at f/u. Based on crude unadjusted comparisons, patients with stable aPL profiles, compared to those with unstable profiles, were more likely to have baseline positive LA test, aCL IgM > 40U (positive trend for IgG), aβ2GPI IgG > 40U (positive trend for IgM), two or more positive aPL tests, and history of arterial events and aspirin use (Table).
Conclusion: In approximately 80% of patients with a baseline clinically significant aPL profile (LA test and/or aCL/aβ2GPI IgG/M >40U), aPL profiles remain consistently significant (stable) during five years of follow-up. Further multivariate analysis will investigate predictors of aPL profile stability over time, and guide future validation studies of stored samples through APS ACTION core laboratories.
To cite this abstract in AMA style:
Gkrouzman E, Sevim E, Finik J, Andrade D, Pengo V, Sciascia S, Tektonidou M, Ugarte A, Chighizola C, Belmont H, Pérez Sánchez L, Ji L, Fortin P, Efthymiou M, De Jesus G, Branch D, Nalli C, Petri M, Cervera R, Rodriguez E, Knight J, Atsumi T, Willis R, Bertolaccini M, Cohen H, Rand J, Erkan D, Of APS ACTION O. Antiphospholipid Antibody Profile Stability over Time: Prospective Results from AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/antiphospholipid-antibody-profile-stability-over-time-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinical-database-and-r/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antiphospholipid-antibody-profile-stability-over-time-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinical-database-and-r/