Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Prior studies indicate that mast cells are involved in chronic inflammation and that their activity in the synovium may contribute to structural progression of osteoarthritis (OA), however the exact role of mast cells in OA remains unclear. Antihistamines act by blocking histamine receptors, and further are found to have anti-inflammatory effects by stabilizing mast cell membranes. Current reports describing antihistamine use in OA patients suggest that antihistamines may reduce development of OA and lead to reduced risk of structural progression. The objective of this analysis was to investigate whether antihistamine use during a two-year trial period was associated with differences in structural progression of OA, as compared with non-use.
Methods: This is a post-hoc analysis of two large phase III trials investigating oral salmon calcitonin in knee OA (NCT00486434 and NCT00704847). To be eligible for study inclusion, patients had to meet the American College of Rheumatology (ACR) criteria for diagnosis of knee OA. The primary outcome measure was structural progression defined as the change in minimum joint-space width measured by use of x-ray imaging from baseline to Year Two. In these trials, participants reported use of antihistamines, defined as medication coded with the ATC code R06A. In our study, we evaluated differences between groups of participants who reported use of antihistamines, versus those who did not, over the 2-year study period. Secondly, the duration of antihistamine use divided into categories of either no use, 1-49, 50-299 or >300 days of use was investigated to evaluate exposure-response relationships. The effect of use of antihistamines was evaluated using ANCOVA analysis adjusting for age, sex, BMI, and baseline JSW.
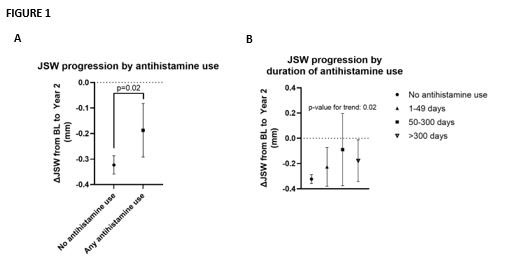
Results: Of a total study population of 2,206 participants, 1,485 completed the trial. Of these, 1,327 were non-users of antihistamines (mean age 64.4 years, 64.1% female, mean BMI 29.0 kg/m2) and 158 reported use of antihistamines of any duration during the trial (mean age 64.5 years, 75.2% female, mean BMI 28.1 kg/m2). Seventy-four participants reported use of antihistamines of a duration between 1-49 days, 21 participants between 50-299 days, and 63 reported use of 300 days or more. As illustrated in Figure 1a, the mean JSW change from baseline in the group of non-users was -0.32 mm (95% CI: -0.36 to -0.29), versus -0.19 mm (95%CI: -0.29 to -0.08, p=0.02 for difference) in the group of patients reporting antihistamine use of any duration. A trend towards an association between duration of antihistamine use and reductions in narrowing of JSW was observed (p for trend: 0.02, Figure 1b).
Conclusion: Use of antihistamines was associated with reduced structural progression in knee OA. Further research evaluating the role of antihistamines in OA is needed to further characterize this observation.
To cite this abstract in AMA style:
Bihlet A, Miller C, Byrjalsen I, Karsdal M, Andersen J, Rao T, Baker M. Antihistamine Use and Structural Progression of Knee OA: A Post-Hoc Analysis of Two Phase 3 Clinical Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/antihistamine-use-and-structural-progression-of-knee-oa-a-post-hoc-analysis-of-two-phase-3-clinical-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antihistamine-use-and-structural-progression-of-knee-oa-a-post-hoc-analysis-of-two-phase-3-clinical-trials/