Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Previous reports have
demonstrated that malondialdehyde-acetaldehyde (MAA) adducts are produced as a
byproduct of oxidative stress and lipid peroxidation and are expressed in RA
joint tissues (Thiele GM, Arthritis Rheumatol 2015). Moreover, MAA adducts co-localize
with citrullinated proteins and promote robust T cell and antibody responses,
the latter being associated with RA disease severity. Whether anti-MAA
antibody concentrations predict treatment response in RA is unknown. The
objective of this study was to examine the role of anti-MAA antibody isotypes
in predicting RA treatment responses in a group of well-characterized patients
participating in a randomized controlled trial.
Methods: As part of a secondary analysis of the
Rheumatoid Arthritis Comparison of Active Therapies (RACAT) trial, banked serum
from baseline, 24, and 48 weeks (n=255) were tested for anti-MAA antibody
isotypes (IgA, IgG, IgM) using ELISA. In the 48-week trial, patients
with active disease despite methotrexate (MTX) were randomized to receive
triple therapy (MTX/sulfasalazine/hydroxychloroquine) or MTX/etanercept. Associations
of treatment response with baseline antibody isotype concentrations and changes
in antibody concentrations at 24 and 48 weeks were examined using non-parametric
Spearman correlations. The primary treatment outcome in this analysis was the
change in DAS28-ESR at 48 weeks.
Results: As previously reported (O’Dell JR, N Engl J
Med, 2013), patients in the RACAT study had a mean age of ~57 years, 46% were
women, and patients had a mean (SD) DAS28 at enrollment of 5.8 (1.9). There
were no associations of baseline anti-MAA antibody concentration with treatment
response at 24 or 48 weeks. Likewise, there were no associations of change in
antibody concentrations with treatment response at 24 weeks. In contrast,
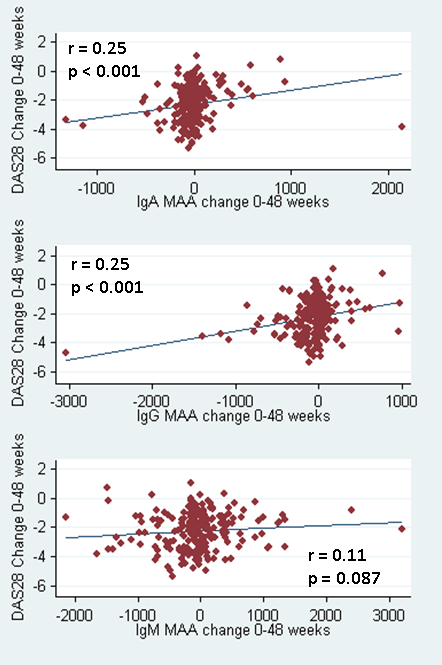
changes in IgA and IgG anti-MAA antibody isotype concentrations from baseline
to 48 weeks were significantly associated with treatment responses (r=0.25;
p<0.001 for both isotypes) (Figure).
Conclusion: Treatment response in RA over one year
of follow-up is strongly associated with declines in both IgA and IgG anti-MAA
antibody isotypes, associations that are not apparent after just 6 months. These
results and prior studies showing the importance of lipid peroxidation in both MAA
adduct formation and RA suggest that anti-MAA antibodies may mediate or act as
a marker of RA disease activity promoted through oxidative stress. Further
studies will be needed to assess whether this relationship is dependent on
specific therapies and to elucidate mechanisms through which reduced anti-MAA
antibodies are associated with improved treatment responses in RA.
To cite this abstract in AMA style:
Mikuls TR, Coburn B, Sayles H, Yu F, Brophy M, O'Dell JR, Klassen LW, Thiele GM. Antibody to Malondialdehyde-Acetaldehyde (MAA) Adducts Serve As Biomarkers of Treatment Response in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/antibody-to-malondialdehyde-acetaldehyde-maa-adducts-serve-as-biomarkers-of-treatment-response-in-rheumatoid-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antibody-to-malondialdehyde-acetaldehyde-maa-adducts-serve-as-biomarkers-of-treatment-response-in-rheumatoid-arthritis/