Session Information
Date: Sunday, October 26, 2025
Title: (0593–0640) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
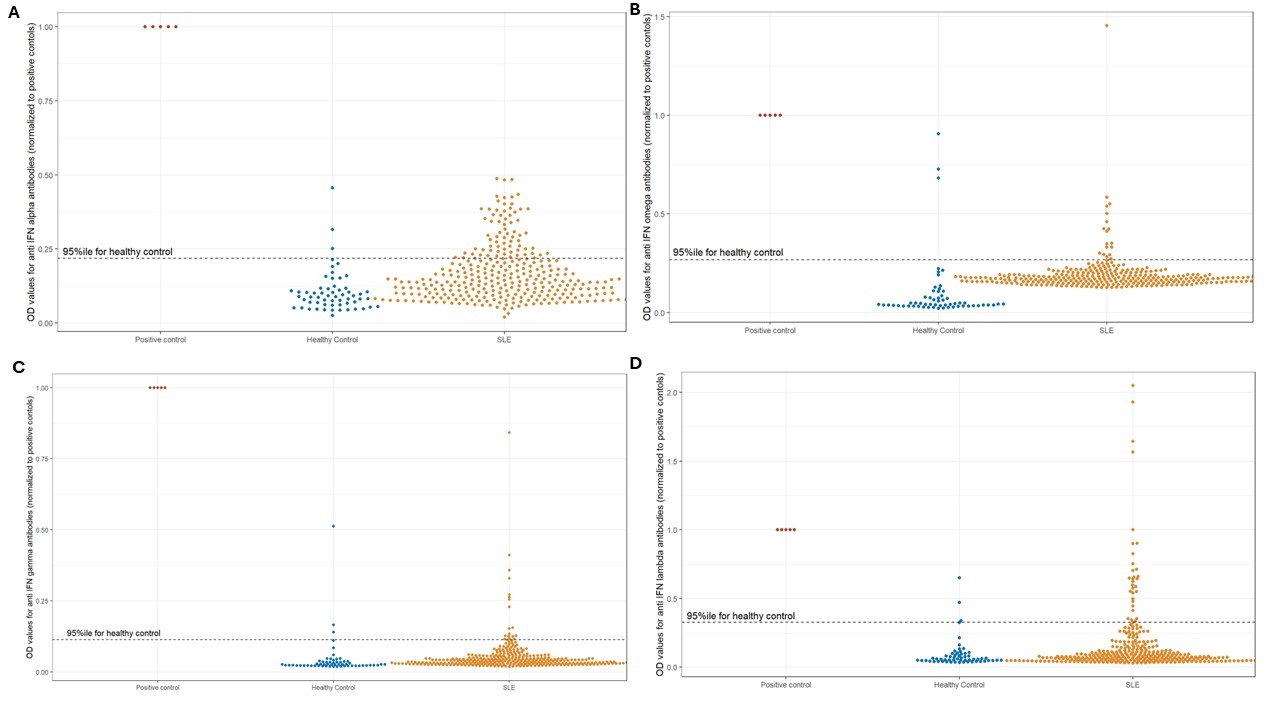
Background/Purpose: Patients with systemic lupus erythematosus (SLE) are predisposed to infections due to immune dysregulation. Autoantibodies to cytokines can cause serious infections, including severe COVID-19, by mycobacteria or other intracellular microbes. We describe here antibodies to type I, II and III interferons (IFNα, ω, γ & λ) as a cause for infections in an inception cohort of SLE (INSPIRE) from India.
Methods: Newly diagnosed SLE (SLICC 2012 criteria) within 6 months of disease onset were enrolled in the INSPIRE cohort from 2018-2023. We developed an ELISA for antibodies to IFN α, ω, γ and λ. Baseline sera retrieved from the biobank were tested for these antibodies. Antibody titres were expressed as optical density (OD) and the 95th percentile of antibody titres in healthy-control population was used to develop cutoffs for positivity. Outcomes studied were infections in the initial 2 years of follow up. Serious infections were those that led to hospitalisation or mortality.
Results: A total of 313 patients of SLE (294 females) with a mean age 27.7±10.2 years were enrolled from 2 centres in North India. In addition, sera from 59 healthy controls were also tested for anti IFN antibodies. Antibodies to IFN-α, IFN-ω, IFN- γ and IFN-λ were present in 73 (23.3%), 18 (5.5%), 18 (5.5%) and 30 (9.6%) patients respectively. Eight patients (2.72%) had more than 1 anti-interferon antibodies. In contrast, 3 (5.08%) healthy controls had antibodies to IFN-α, IFN-ω, IFN- γ and IFN-λ each.In the initial 2 years, there were 107 infections, of which 31 were serious infections. Bacterial including mycobacterial infections (79, 25.2%) were most common followed by viral (32, 10.2%) and fungal (15, 4.8%). Herpes zoster (25, 7.9%) was the most common infection followed by tuberculosis (11, 3.5%). No healthy control had history of serious infections.Antibodies to IFN-α were associated with serious infections (OR 2.79, 1.29-6.04), tuberculosis (OR 3.46, 1.03-11.68), viral infections (OR 2.64, 1.23-5.68) and Herpes zoster (OR 3.1, 1.2-8.02), but not with overall infections (OR 1.3, 0.76-2.35), bacterial (OR 1.27, 0.69-2.36) or fungal (OR 1.23, 0.38-3.99) infections. Antibodies to IFN-λ were associated with serious infections (OR 2.93, 1.1-7.83) but not with any subtype of infection. Antibodies to IFN-ω and IFN-γ were not associated with any higher risk of infections.
Conclusion: Antibodies to IFN-α are the most common anti-interferon antibody in an Indian inception cohort of SLE and predispose to serious infections, especially viral infections, Herpes zoster and tuberculosis in the initial 2 years of follow-up.
To cite this abstract in AMA style:
Chatterjee R, Singh K, Gupta R, Sinha S, Aggarwal A. Antibodies to Type I and Type III Interferons at Diagnosis Predispose to Serious Infections on Follow Up in an Inception cohort of SLE (INSPIRE) from India. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/antibodies-to-type-i-and-type-iii-interferons-at-diagnosis-predispose-to-serious-infections-on-follow-up-in-an-inception-cohort-of-sle-inspire-from-india/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antibodies-to-type-i-and-type-iii-interferons-at-diagnosis-predispose-to-serious-infections-on-follow-up-in-an-inception-cohort-of-sle-inspire-from-india/