Session Information
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: There are concerns about using immunosuppressive agents for treatment of rheumatic diseases in patients with HIV infection due to concerns of opportunistic infection. We previously reported a series of eight HIV positive patients primarily seen at a County Outpatient Rheumatology Clinic treated with anti-TNF agents between 2003 and 2008 (Cepeda et al, Ann Rheum Dis 67: 710-12, 2008). The purpose of this study is to report the safety and efficacy of anti-TNF agents in these patients over time to the present and to update our experience with biologic agents in this setting in additional patients.
Methods: All but three of the patients were seen at the Harris County HIV Outpatient Clinic. At baseline visit, sociodemographic characteristics, CD4 counts, HIV viral load and medications were collected. Patients with rheumatoid arthritis or spondyloarthritis were initially treated with NSAIDs and nonbiologic disease modifying antirheumatic drugs(DMARDs) (methotrexate, sulfasalazine) and intra-articular/intralesional corticosteroids. Those patients with persistent disease activity despite conservative therapy were begun on anti-TNF agents, provided CD4 counts were above 200 cells/μl and HIV viral load < 60,000 copies/ml (as per guidelines for use of immunosuppressive agents in the face of HIV infection).
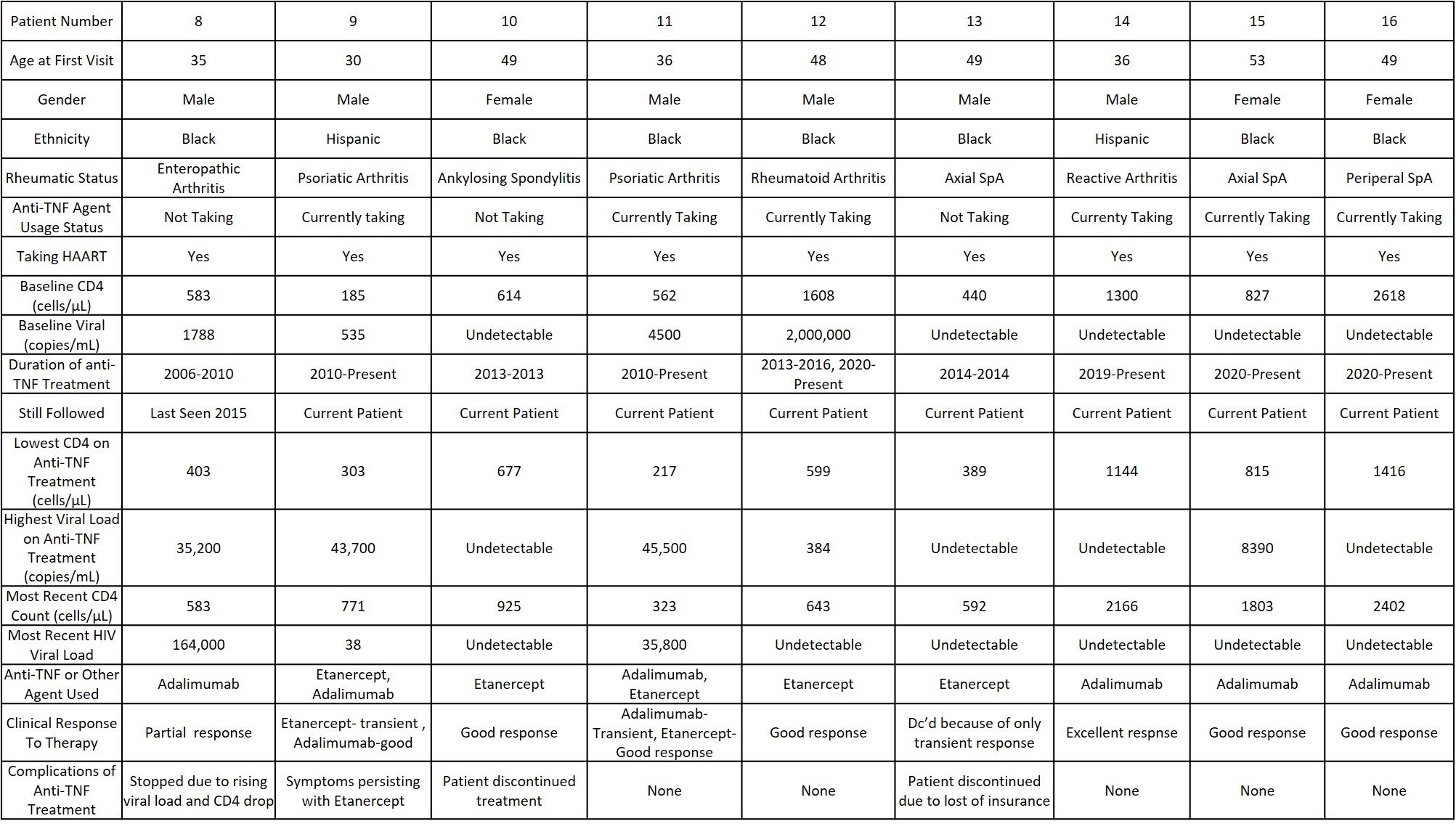
Results: In total, 18 patients were treated with anti-TNF agents, including 8 treated with anti-TNF agents between 2003 and 2006 (two RA, two peripheral spondyloarthritis (SpA), of whom we have follow-up data on seven, and an additional nine treated with anti-TNF agents since then. There were no major infectious episodes that necessitated discontinuation of the medications, although one patient seen after 2006 had his adalimumab discontinued by his primary care doctor because of rising HIV viral load. The data on seven of the original eight are shown in Table 1 (one patient was seen only transiently in 2004 and was lost to followup).Of these original seven patients, four are still currently followed (of whom one is on adalimumab and one ustekinumab, the other two in remission) and all seven have been seen in the past six years. Of the nine patients begun on anti-TNF agents since 2006, all but one are currently being followed. by us, of whom six are still taking anti-TNF agents. Three had discontinued them, one due to lack of efficacy, one because of a rising HIV viral load, and one due to fears of toxicity(though no actual event was recorded)..
Conclusion: In this series of 17 HIV positive patients, followed up to 18 years, the largest and longest followed reported to date, our data underscore the safety of using anti-TNF agents in patients with HIV infection over long duration, provided standard precautions of using immunosuppressive agents in the setting of HIV infection are followed.
To cite this abstract in AMA style:
Naovarat B, Williams F, Salazar G, Reveille J. Anti-TNF Usage in Patients with HIV Infection 2003-2021: Long Term Safety and Followup [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/anti-tnf-usage-in-patients-with-hiv-infection-2003-2021-long-term-safety-and-followup/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-tnf-usage-in-patients-with-hiv-infection-2003-2021-long-term-safety-and-followup/