Session Information
Date: Sunday, November 17, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-synthetase syndrome (ASSD) is a subset of idiopathic inflammatory myopathy characterized by autoantibodies directed against aminoacyl tRNA synthetases. Interstitial lung disease (ILD) can be the first and most prominent feature of ASSD. Our current study aims to describe the characteristics of ASSD-related interstitial lung disease (ASSD-ILD) in comparison to non-ASSD-related ILDs.
Methods: We utilized the Classification Criteria for Anti-synthetase Syndrome (CLASS) project database, which has physician-reported data from 92 centers across 30 countries, to identify patients with ASSD-ILD (cases) and ILD related to ASSD mimicking conditions (controls). Demographic, clinical, radiographic, and physiological data were compared between cases and controls using chi-square tests for categorical variables and Student’s t-test for continuous variables. The association of cases (versus controls) with all-cause mortality was analyzed using logistic regression adjusted for follow-up time.
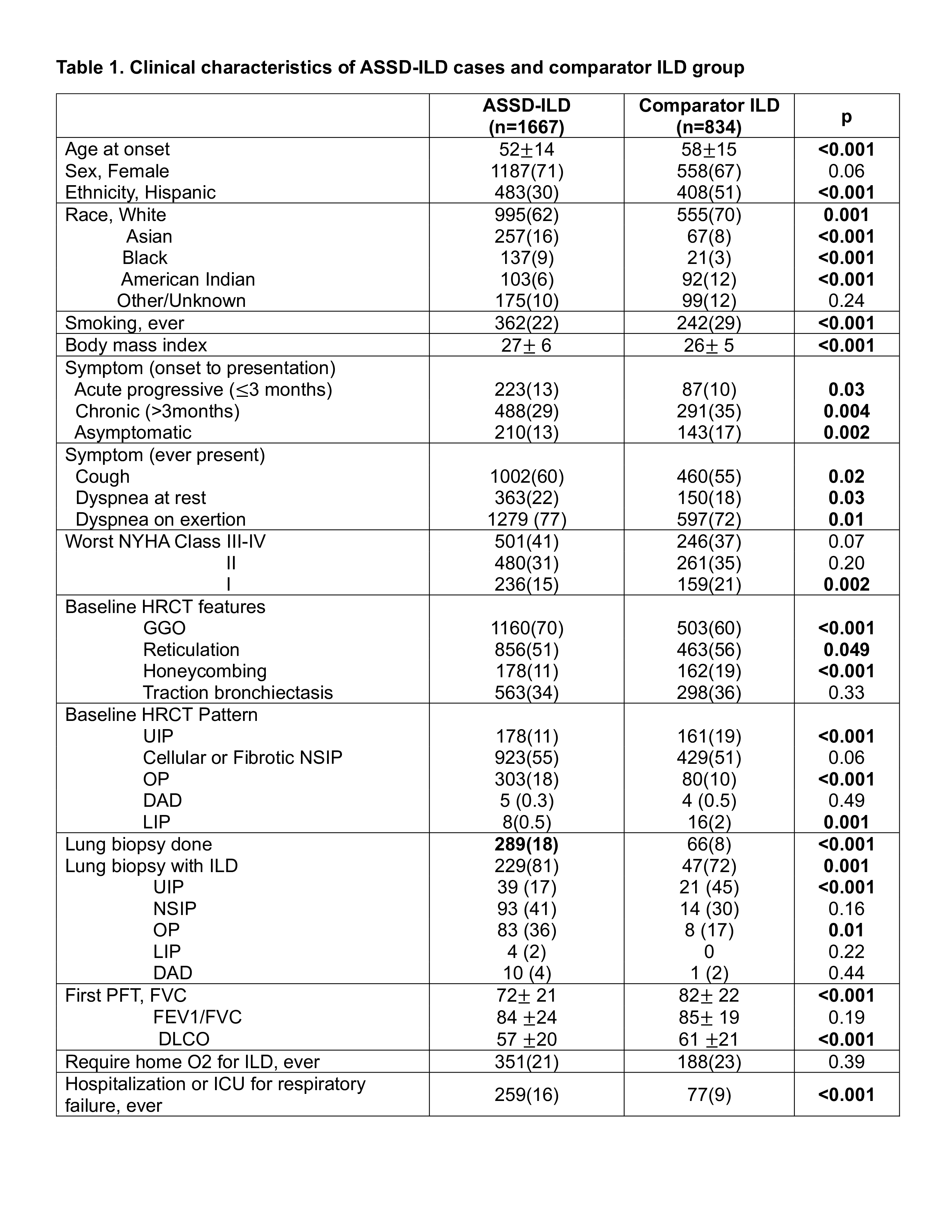
Results: A total of 2501 patients with ILD were identified, 1667 cases and 834 controls (Table 1). The comparator group included patients with dermatomyositis (15%), systemic sclerosis (18%), rheumatoid arthritis (14%), interstitial pneumonia with autoimmune features without anti-synthetase antibodies (IPAF, 20%), idiopathic pulmonary fibrosis (8%) and other mimicking conditions (24%). ASSD-ILD patients were younger at disease onset and included fewer Hispanics.
ASSD-ILD cases were more likely to present with acute progressive disease (within 3 months) and were more often symptomatic with cough and dyspnea. The controls were more likely to present with chronic, asymptomatic disease.
The pulmonary function tests at diagnosis showed significantly lower forced vital capacity and diffusing capacity of carbon monoxide in ASSD-ILD compared to controls. The most common ILD pattern on high-resolution chest computed tomography (HRCT) at the time of diagnosis was the nonspecific interstitial pneumonia (NSIP) pattern for both groups. Organizing pneumonia (OP) was more common in ASSD-ILD, while the usual interstitial pneumonia (UIP) pattern was more common in controls. ASSD-ILD HRCT scans had more ground glass, while controls had more reticulation and honeycombing.
ASSD-ILD patients had significantly higher rates of hospitalization for respiratory failure. Survival data was available in 65% of the dataset (60% of cases and 74% in controls) over a follow-up time of 4 [2-8] years, median [interquartile range]. All-cause mortality was 7.7% in ASSD-ILD patients and 4.3% in controls (p=0.004). ASSD-ILD had an increased odds for death compared to controls, which remained significant after adjustment for follow-up time (OR [95%CI] 2.36 [1.42-3.93], p=0.0004).
Conclusion: Patients with ASSD-ILD were more likely to have symptomatic ILD and a more acute presentation, and had higher rates of hospitalization compared to patients with non-ASSD-related ILD. Higher odds of all-cause mortality in ASSD-ILD cases compared to comparators highlight the importance of early recognition and the need for future work to identify poor prognostic factors in these patients.
Abbreviations: NYHA, New York Heart Association functional class; HRCT, high resolution computed tomography; UIP, Usual interstitial pneumonia; NSIP, Nonspecific interstitial pneumonia; OP, organizing pneumonia; DAD, diffuse alveolar damage; LIP, lymphoid interstitial pneumonia; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; ICU, intensive care unit
To cite this abstract in AMA style:
Bae S, Bozan F, Rivero Gallegos D, Zanframundo G, Faghihi-Kashani S, Ventura i, Dourado E, Loganathan A, sambataro G, Yoshida A, Bonella F, J Corte T, J Doyle T, fiorentino d, Gonzalez-Gay M, Hudson m, Kuwana M, Notarnicola A, Mammen A, McHugh N, Miller F, Montecucco C, Oddis C, Rojas-Serrano J, Schmidt J, Scire C, Selva-O’Callaghan A, Werth V, Cavagna L, Aggarwal R. Anti-synthetase Syndrome (ASSD) Related Interstitial Lung Disease (ILD) in Comparison to Non-ASSD Related ILDs: Analysis from the “Classification Criteria for Anti-synthetase Syndrome (CLASS)” Project Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anti-synthetase-syndrome-assd-related-interstitial-lung-disease-ild-in-comparison-to-non-assd-related-ilds-analysis-from-the-classification-criteria-for-anti-synthetase-syndrome-class/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-synthetase-syndrome-assd-related-interstitial-lung-disease-ild-in-comparison-to-non-assd-related-ilds-analysis-from-the-classification-criteria-for-anti-synthetase-syndrome-class/