Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: People with immune-mediated inflammatory diseases (IMIDs) may be more vulnerable to severe COVID-19 outcomes. COVID-19 vaccination is a key element in mitigating this risk. Anti-SARS-CoV-2 antibodies (Ab), including anti-spike (S) and anti-receptor binding domain (RBD) Ab, are metrics of seroconversion following COVID-19 vaccination in the general population. We assessed if anti-S and anti-RBD antibodies were negatively correlated with COVID-19 infection in individuals with IMIDs.
Methods: SUCCEED, a prospective Canada-wide study, was conducted in two phases. First, between Feb 2021 – Jul 2023, adult IMID participants provided dried blood spot samples for anti-S and anti-RBD ELISA testing at intervals of 1, 3, 6, and 12 months following each COVID-19 vaccine dose. Second, between Sep 2022 – Aug 2023, consenting participants from 4 centres also provided monthly saliva samples for PCR detection of SARS-CoV-2. We studied subjects receiving at least their primary series (3+ doses) of a COVID-19 vaccine.
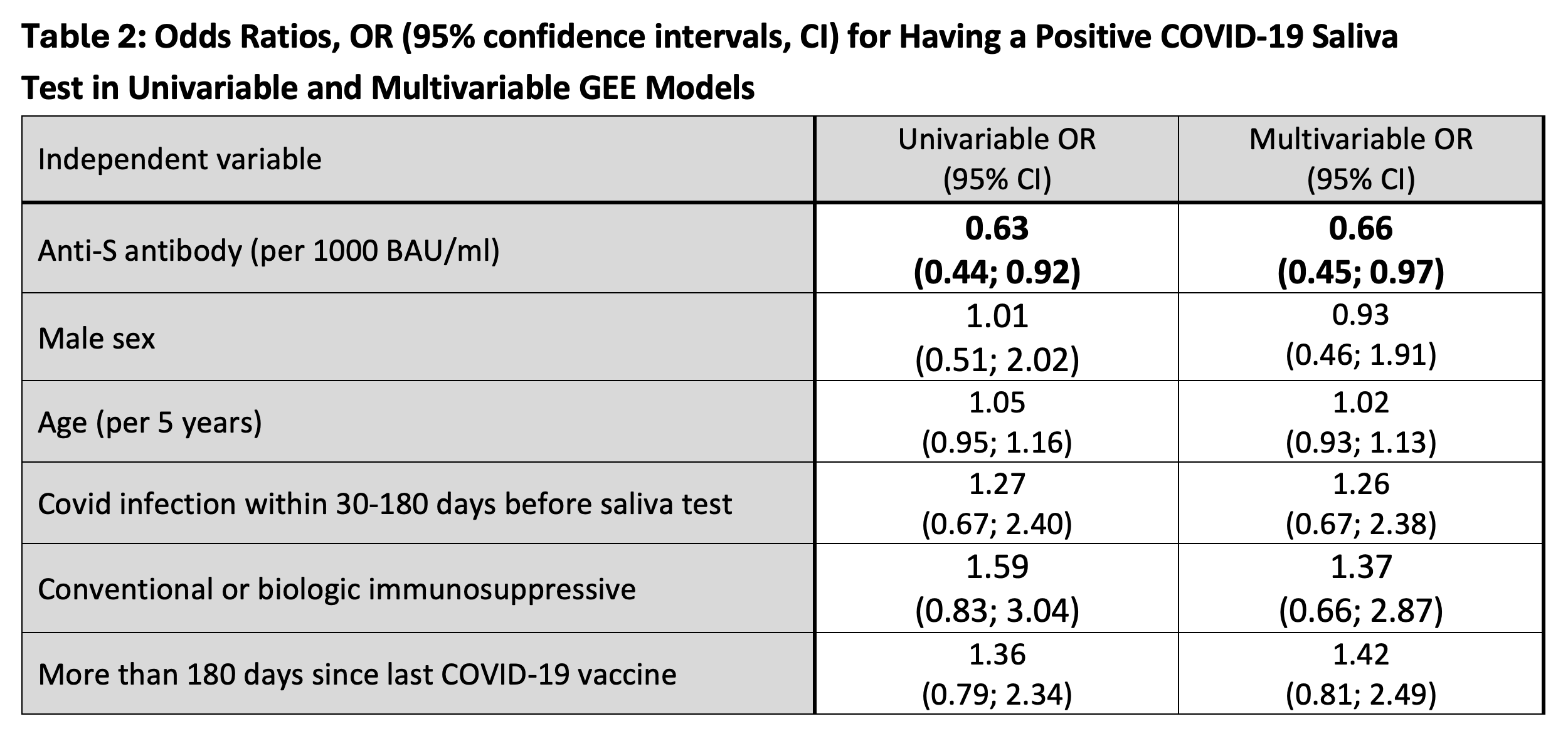
Multivariable general estimating equation (GEE) models (accounting for repeated measures) evaluated PCR SARS-CoV-2 detection in saliva, assessing the effects of anti-S or anti-RBD levels (in separate models) within the 6 months preceding a given saliva sample. We controlled for recent COVID-19 infection, sex, age, medications (conventional immunosuppressives, biologics, and prednisone), and time since last COVID vaccine.
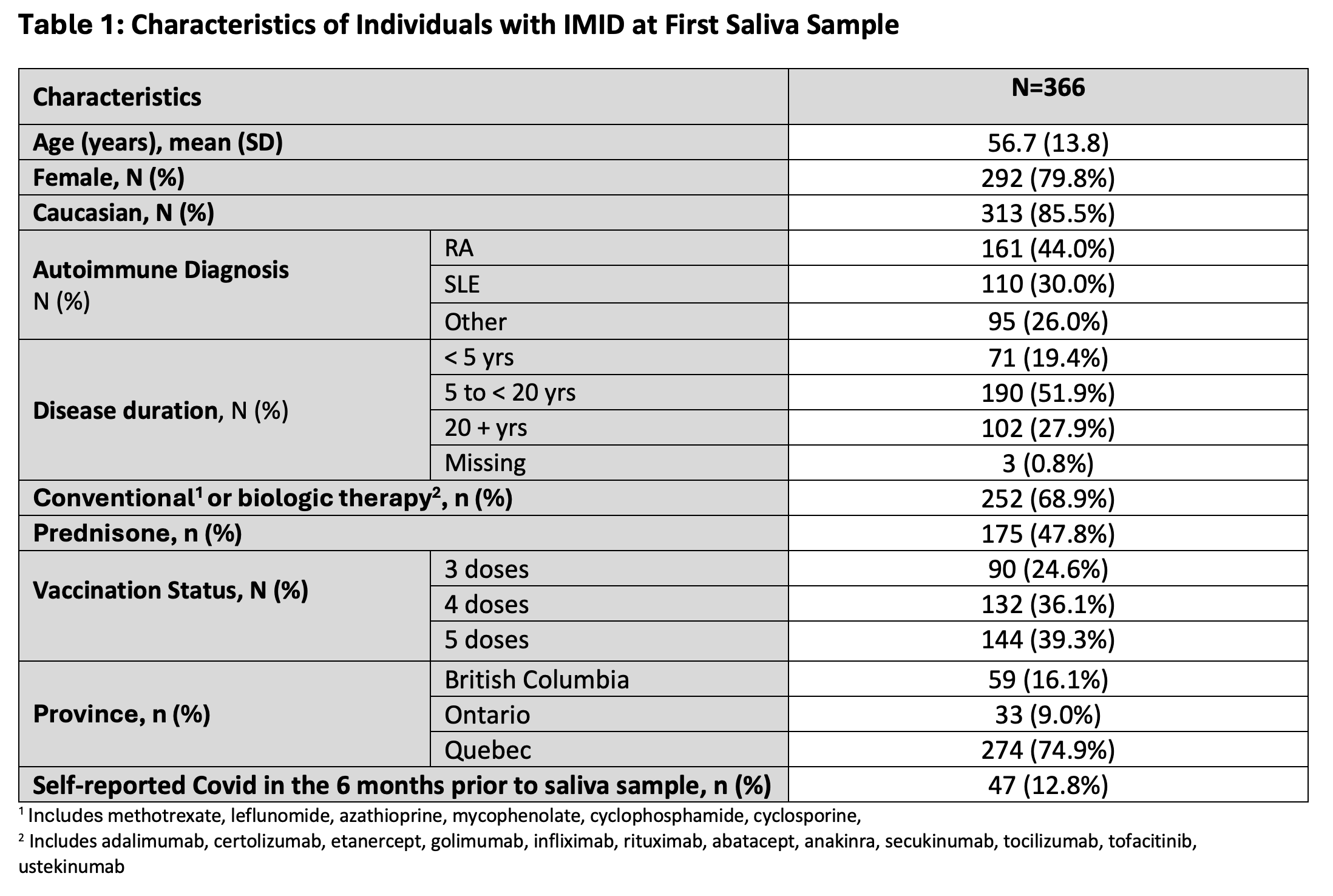
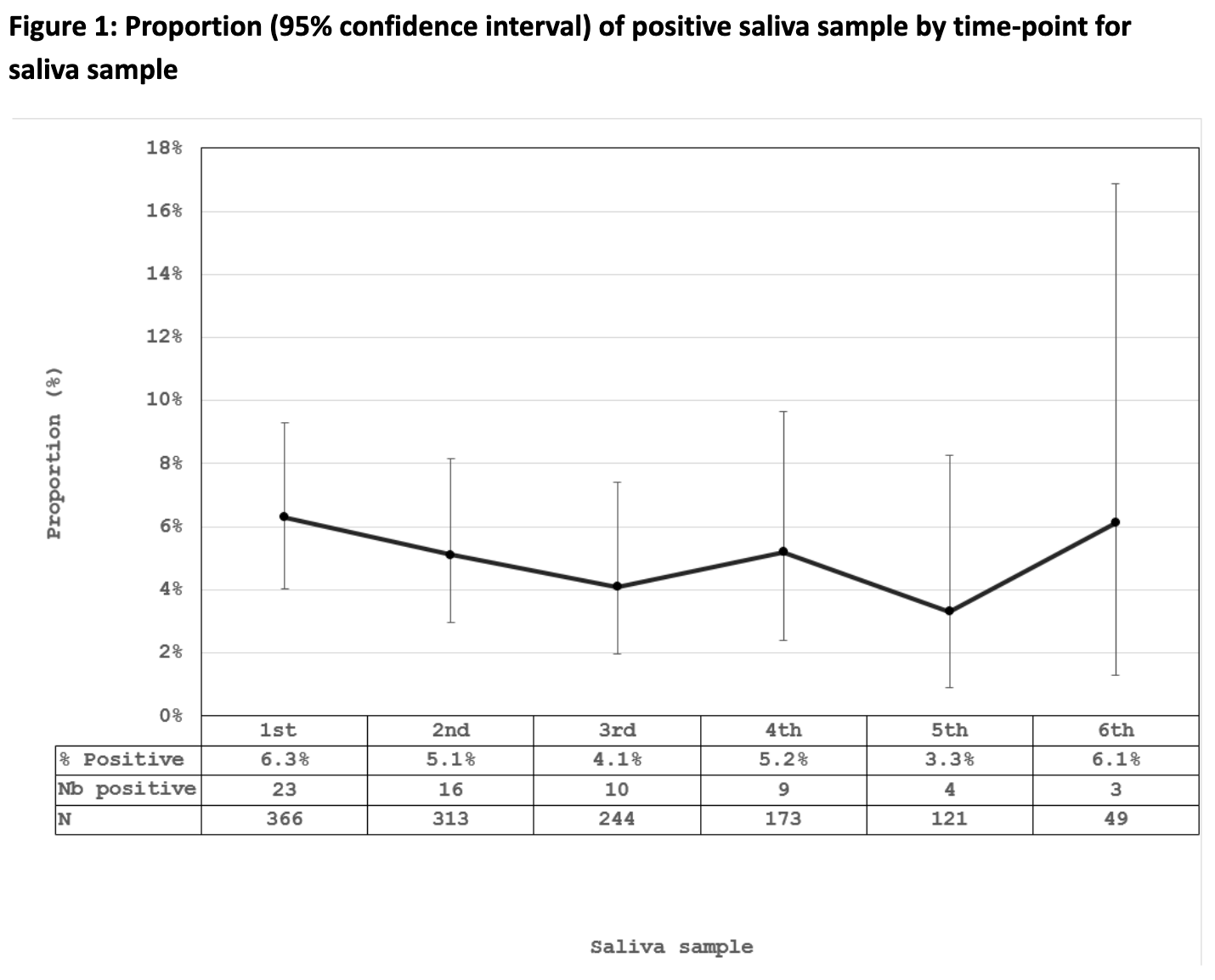
Results: 366 participants contributed 1,266 saliva samples. Participants were 79.8% female and 85.5% White, with median age 56.7 (standard deviation: 13.8) years. Most participants were taking immunosuppressants (N=252, 68.9%). The majority (N=356, 97.3%) of participants displayed seroconversion at the first saliva sample, defined as ≥11.3 Binding Antibody Units (BAU)/ml for anti-S or ≥31 BAU/ml for anti-RBD.
In the GEE models of positive saliva PCR for SARS-CoV-2, a 1000 BAU/ml increase in anti-S was associated with an adjusted odds ratio (aOR) of 0.66 (95% confidence interval [CI] 0.45-0.97). Anti-RBD Ab levels had a similar effect (aOR 0.91, 95% CI 0.81-1.02).
Conclusion: In this large, multi-centre sample of COVID-19-vaccinated individuals with IMIDs, most of whom were immunosuppressed, we demonstrated that anti-S Ab levels were associated with lower odds of positive saliva PCR test for SARS-CoV-2, with a similar trend for anti-RBD Ab. This highlights clear benefits for vaccination against SARS-CoV-2 to prevent COVID-19 disease in individuals with IMIDs.
To cite this abstract in AMA style:
Tan J, Avina-Zubieta J, Fortin P, Gingras A, Larche M, Bowdish D, Berger C, Colmegna I, Hitchon C, Lacaille D, Richards D, Lalonde N, Kirmani A, Lee J, Bernatsky S. Anti-Spike Antibodies Protect Against COVID-19 Infection in Immune-Mediated Inflammatory Diseases: Findings from the SUCCEED Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anti-spike-antibodies-protect-against-covid-19-infection-in-immune-mediated-inflammatory-diseases-findings-from-the-succeed-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-spike-antibodies-protect-against-covid-19-infection-in-immune-mediated-inflammatory-diseases-findings-from-the-succeed-study/