Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Due to better effectiveness and longer drug survival, a TNF inhibitor (TNFi) should preferably be combined with methotrexate (MTX). MTX has been found to reduce the development of anti-drug antibodies (ADAs), which decreases the risk of treatment failure. ADA formation, however, predominately occurs during treatment initiation. To our knowledge, the effect of tapering or stopping MTX on the formation of ADAs after longstanding treatment with TNFi is not yet known. Therefore, the aim of this study was to assess the effect of tapering and stopping MTX on the formation of ADA in rheumatoid arthritis (RA) patients with longstanding use of adalimumab (ADL).
Methods: For this study we used data from the TApering strategies in Rheumatoid Arthritis (TARA) trial. The TARA trial was a multicenter, single-blinded randomized trial that included established RA patients with a well-controlled disease, defined as a disease activity score (DAS) ≤2.4 and a swollen joint count ≤1, which was achieved with conventional synthetic (cs)DMARDs and a TNFi. Eligible patients were randomized into gradual tapering csDMARD (mainly methotrexate) followed by the TNFi, or vice versa. Tapering of MTX was realized by cutting the dosage into half, a quarter and thereafter it was stopped. The TNF inhibitor was tapered by doubling the dose interval, followed by cutting the dosage into half, and thereafter it was stopped. The total tapering schedule took 6 months, with dose adjustments every 3 months as long as there was still a controlled disease. ADA and ADL serum levels were measured at each 3-monthly visit, if serum samples were available, by a drug tolerant enzyme-linked immunosorbent assay.
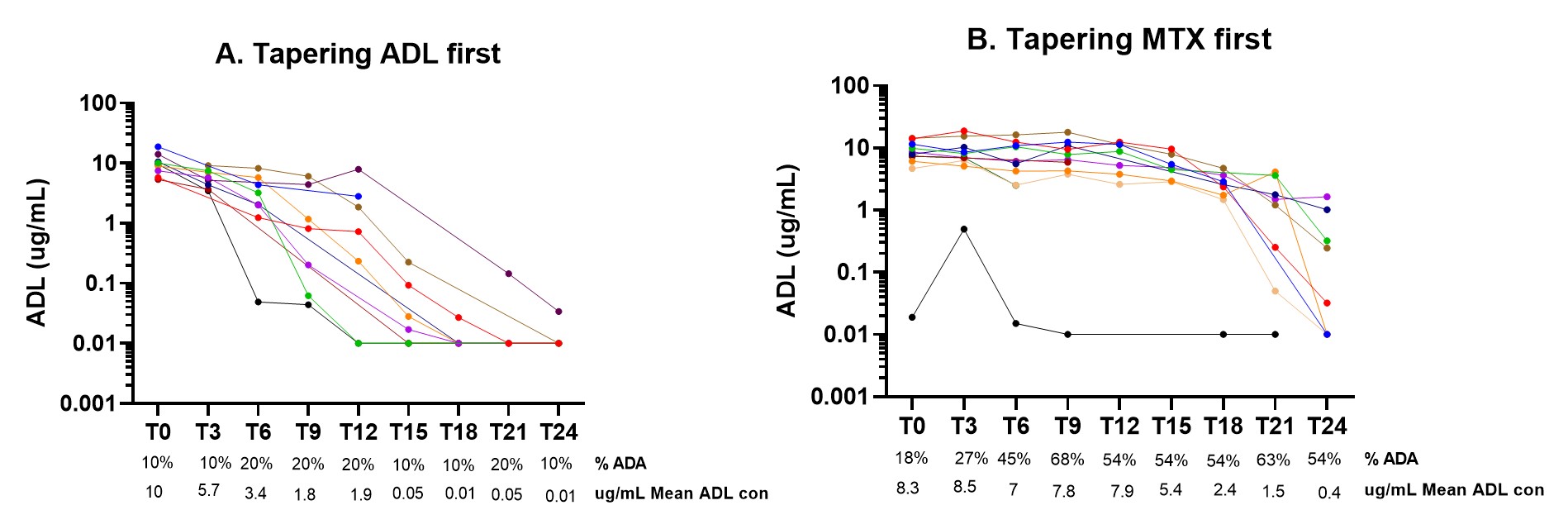
Results: Of the 46 included RA patients, 21 patients did not experience a disease flare. Among patients who did not experience a flare, 10 patients tapered ADL first, while 11 patients tapered their csDMARD first. Patients had an average symptom duration of 6,5 years and were predominantly female 71% with an average age of 54 years. At baseline, the DAS (standard deviation) was 1,0 (0,6) and the mean MTX dosage before tapering was 16.5 mg/week. In the group tapering ADL first, detectable ADA prevalence remained unchanged between 10 to 20 % (Figure 1A). On the other hand ADA detection in serum increased from 18% to 68% in the RA patients who were tapering and stopping the MTX first (Figure 1B). Despite the increase in ADA formation, the mean ADL concentration showed a minor decrease from 8.3 (T0) to 7,8 (T9) ug/mL (Figure 1B). In the second year, when also ADL is tapered, patients with detectable ADA seems to have faster clearance of ADL (figure 1B).
Conclusion: Our data shows that there is an increase in detectable ADA after tapering and stopping MTX in patients with longstanding use of ADL, but this seems not to have a major effect on the clearance of ADL in the standard dose.
Abbreviations: ADL, adalimumab; con., concentration; ADA, anti-drug antibodies
To cite this abstract in AMA style:
Layegh Z, de Jong P, Atiqi S, loeff F, Dijk l, vaz G, Dolhain r, Rispens T, Wolbink G. Anti-drug Antibodies Formation During Tapering and Stopping of Methotrexate in Rheumatoid Arthritis Patients with Longstanding Use of Adalimumab [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/anti-drug-antibodies-formation-during-tapering-and-stopping-of-methotrexate-in-rheumatoid-arthritis-patients-with-longstanding-use-of-adalimumab/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-drug-antibodies-formation-during-tapering-and-stopping-of-methotrexate-in-rheumatoid-arthritis-patients-with-longstanding-use-of-adalimumab/