Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Clinical Aspects - Poster I: Clinical Characteristics/Presentation/Prognosis
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Current clinical guidelines recommend testing for anti-citrullinated protein antibodies (ACPA) at the time of RA diagnosis.1 However, there is a lack of information about the frequency of ACPA testing at RA diagnosis or thereafter. The aim of this study was to describe the frequency of ACPA testing over time and to investigate the potential differences in demographics, co-morbidities and hospitalizations between patients (pts) who received ACPA tests versus those who did not.
Methods: Data from the three following US commercial healthcare claims databases (db) were analyzed: IMS PharMetrics Plus (db A), Optum Clinformatics Data Mart (db B) and Optum Clinformatics Data Mart Medicare (db C). Pts with two diagnosis codes for RA from January 1 2007 to December 31 2014, treatment with a DMARD, and continuous enrolment for at least 12 mths before and 6 mths after the index date were included. ACPA and RF testing claims identified by current procedural terminology codes were included in the analysis. ACPA testing rates were evaluated as number of pts with tests divided by total number of pts with and without tests. Multinomial logistic regression was used to evaluate baseline covariates associated with single ACPA test before the index date, single ACPA test after the index date and multiple ACPA tests.
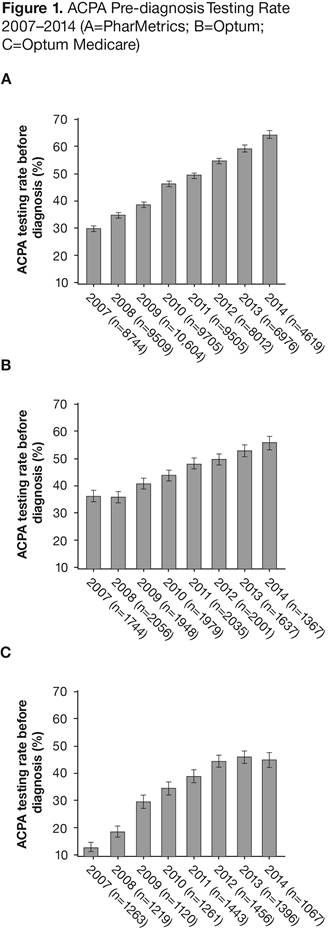
Results: 67,674 newly diagnosed RA pts in db A (age 18–64 yrs), 14,767 in db B (age 18–64 yrs) and 10,225 in db C (age ≥65 yrs) met the study inclusion criteria. The overall ACPA testing rate (95% CI) was 70.6% (70.3, 70.9) in db A, 72.2% (71.5, 72.9) in db B and 63.5% (62.5, 64.4) in db C. The ACPA testing rates increased from 2007 to 2014 for all three db (Figure). The corresponding RF testing rates were 75.4% (75.1, 75.7), 85.1% (84.5, 85.6) and 77.8% (77.0, 78.6), respectively. Pts tested and not tested had similar characteristics, with a standardized difference of >0.10 in only a few co-morbidities. The odds ratio (OR) for pre-diagnosis testing for each year increase from 2007 to 2014 was 1.26 (95% CI: 1.25, 1.28) in db A, 1.19 (1.17, 1.22) in db B and 1.27 (1.23, 1.31) in db C. Women were more likely to have multiple tests in db A (OR: 1.15; 95% CI: 1.08, 1.21) and in db B (OR: 1.29; 95% CI: 1.14, 1.45), but not in db C (OR: 0.93; 95% CI: 0.80, 1.08). Pts who received ACPA tests, vs those who did not, had a lower rate of 1-yr hospitalization; the hazard ratio (95% CI) after adjustment for covariates was 0.76 (0.71, 0.80) in db A, 0.86 (0.75, 0.98) in db B and 0.76 (0.68, 0.85) in db C.
Conclusion: The ACPA testing rate, especially pre-diagnosis testing, increased significantly from 2007 to 2014. Women aged 18–64 yrs were more likely to have multiple tests. A slightly lower rate was seen in RA pts ≥65 yrs old.2 1. Aletaha D, et al. Ann Rheum Dis 2010;69:1580–8. 2. Original abstract © EULAR/BMJ. First presented at EULAR 2016 and published in Ann Rheum Dis 2016;75 (Suppl 2):1020. Any reprints, promotional options, education material etc have to be done through the original source (ARD/BMJ).
To cite this abstract in AMA style:
Alemao E, Guo Z, Burns L. Anti-Citrullinated Peptide Antibodies Testing Rate over Time in Newly Diagnosed RA Patients – Data from Three Administrative Claims Databases (2007–2014) [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/anti-citrullinated-peptide-antibodies-testing-rate-over-time-in-newly-diagnosed-ra-patients-data-from-three-administrative-claims-databases-2007-2014/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-citrullinated-peptide-antibodies-testing-rate-over-time-in-newly-diagnosed-ra-patients-data-from-three-administrative-claims-databases-2007-2014/