Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: In 2 phase 3 trials, TULIP-1 and TULIP-2, anifrolumab, a type I IFN receptor mAb, improved disease activity in patients with SLE.1,2 Here, we compared the efficacy of anifrolumab in patients with recent onset vs established SLE disease (defined by time since diagnosis), using pooled data from the TULIP trials.
Methods: TULIP-1 (NCT02446912) and TULIP-2 (NCT02446899) were randomized, placebo-controlled, 52-week trials of intravenous anifrolumab 300 mg every 4 weeks for 48 weeks in eligible patients who fulfilled the ACR 1997 criteria for SLE and had moderate to severe SLE despite standard therapy.1,2 Baseline characteristics and BILAG–based Composite Lupus Assessment (BICLA)3 response rates at Week 52 for anifrolumab 300 mg vs placebo were compared between patients who at the time of their baseline study visits were within 2 years of their SLE diagnosis (recent onset) and patients who were diagnosed beyond 2 years (established). Efficacy was analyzed with a stratified Cochran–Mantel–Haenszel approach controlling for randomization stratifications factors and study.
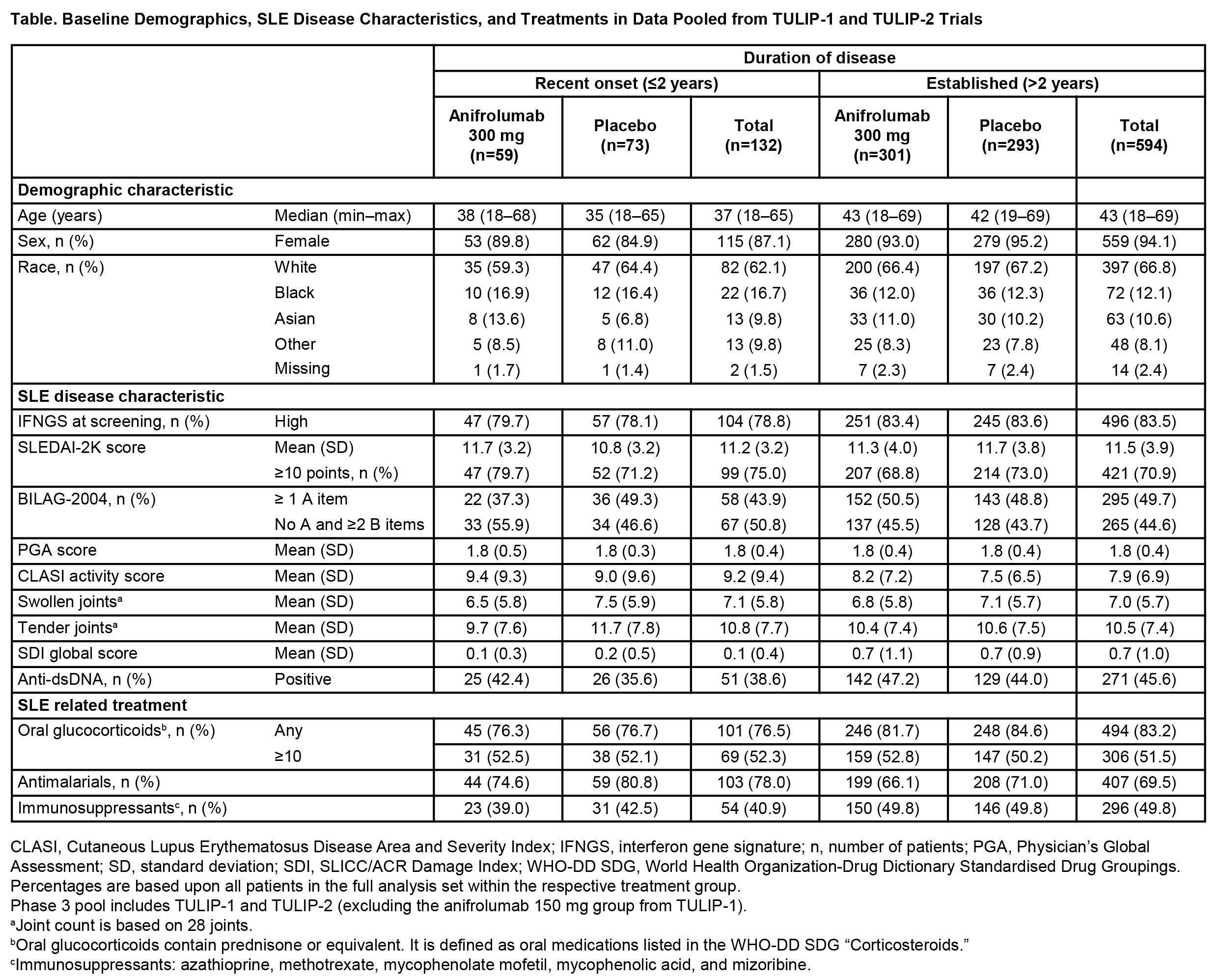
Results: Of the 726 patients included from TULIP-1 and TULIP-2 (anifrolumab, n=360; placebo, n=366), 594 had established disease (anifrolumab, n=301; placebo, n=293), and 132 had recent onset disease (anifrolumab, n=59; placebo, n=73) at baseline. In contrast to patients with recent onset disease, patients with established disease had a higher median age (43 vs 37 years), were more likely to be female (94.1% vs 87.1%), and less likely to be black (12.1% vs 16.7%). At baseline, patients with established disease were more likely to be IFN gene signature high (83.5% vs 78.8%), anti-dsDNA antibody-positive (45.6% vs 38.6%), have ≥1 BILAG-2004 A item (49.7% vs 43.9%), have a higher mean global SDI score (0.7 vs 0.1), and be receiving oral glucocorticoids (83.2% vs 76.5%), and immunosuppressants (49.8% vs 40.9%), but not anti-malarials (69.5% vs 78.0%) (Table). The numbers of BILAG-2004 A or B items across organ domains at baseline were comparable in patients with established or recent onset disease, excluding the renal domain, where a higher proportion of patients with established disease had more severe scores (A or B items; 8.9% vs 3.0%) (Figure). Treatment benefit of anifrolumab vs placebo, assessed by BICLA response at Week 52, was observed in patients with established (difference [95% CI] 17.1% [9.3–24.8], nominal P<0.001) and recent onset disease (difference [95% confidence intervals (CI)] 14.4% [−2.2–31.1], nominal P=0.090).
Conclusion: Data from the TULIP trials support the efficacy of anifrolumab in patients with SLE who have either established or recent onset disease.
1. Furie RA. Lancet Rheumatol. 2019;1:e208–19.
2. Morand EF. N Engl J Med. 2020;382:211–21.
3. Wallace DJ. Ann Rheum Dis. 2014;73:183–90.
To cite this abstract in AMA style:
Kalunian K, Dall'Era M, Furie R, Morand E, Psachoulia K, Maho E, Lindholm C, Tummala R. Anifrolumab Results in Favorable Responses Regardless of SLE Disease Duration: Post Hoc Analysis of Data from 2 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/anifrolumab-results-in-favorable-responses-regardless-of-sle-disease-duration-post-hoc-analysis-of-data-from-2-phase-3-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anifrolumab-results-in-favorable-responses-regardless-of-sle-disease-duration-post-hoc-analysis-of-data-from-2-phase-3-trials/