Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The assessment of radiographic data from long-term studies in patients (pts) with rheumatoid arthritis (RA) poses a significant challenge, given the potential involvement of multiple readers who typically evaluate films from only a subset of the available time points, often re-scoring previously read images. This analysis describes an integration approach to evaluate the complete set of radiographic scores assessed over several years (yrs) from long-term studies of adalimumab (ADA).
Methods: Data from 2 large, multicenter, phase 3, randomized, placebo (PBO)-controlled trials of ADA were analyzed: PREMIER (MTX-naïve pts, early RA1), had a 2-yr double-blind (DB) period followed by an ongoing 8-yr open-label extension (OLE); DE019 (MTX-inadequate responders, long-standing RA2), had a 1-yr DB period followed by a completed 9-yr OLE. Pts received OL ADA±MTX in both OLEs. This post hoc analysis evaluated radiographic data based on randomization to the original PBO+MTX and standard dose ADA+MTX arms through 8 yrs of treatment in PREMIER and 10 yrs in DE019. Radiographic progression was assessed using the change in modified total Sharp score (ΔmTSS) from baseline (BL). Radiographs were assessed at Yrs 2, 3, 5, and 8 (PREMIER) and Yrs 1, 2, 3, 5, 6, 8, and 10 (DE019). At each assessment yr, radiographs from BL and selected prior yrs were re-read. A mixed effect model was used to evaluate the repeated measurements at different time points within different assessment yrs in the integrated analysis. ΔmTSS at each time point was estimated by least square mean and summarized alongside the most recent assessment yr of PREMIER (Yr 8, which included repeat reads for BL, and Yrs 2 and 6) and DE019 (Yr 10, which included repeat reads for BL, and Yrs 1 and 8).
Results: Radiographic data from 452 pts in PREMIER (215, PBO+MTX; 237, ADA+MTX) and 327 pts in DE019 (162, PBO+MTX; 165, ADA+MTX) with BL and ≥1 post-BL radiograph were identified. Radiographic progression was most pronounced in pts receiving PBO+MTX during the DB periods, but progression slowed dramatically upon switch to OL ADA±MTX therapy in both trials (Figure). Following up to 8 yrs of treatment, pts in PREMIER experienced ΔmTSS estimates of 11.1 (PBO+MTX) and 3.9 (ADA+MTX) units; pts in DE019 experienced estimates of 6.6 (PBO+MTX) and 0.9 (ADA+MTX) units through up to 10 yrs of treatment. The estimated curves in each of the studies revealed subtle changes in progression rates not seen in their respective most recent assessment yr.
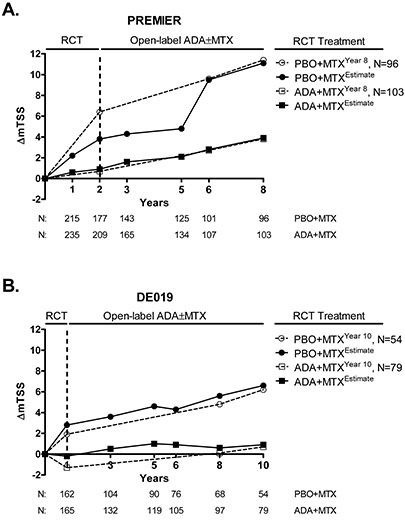
Conclusion: Longitudinal, data integration analyses factoring in mTSS from all available assessments enabled a robust estimate of total radiographic progression in 2 long-term studies of ADA±MTX. Moreover, the present analysis confirmed the radiographic efficacy of long-term therapy with ADA±MTX.
Reference: 1Arthritis Rheum 2006;54:26–37; 2Arthritis Rheum 2004;50:1400–11.
Disclosure:
D. van der Heijde,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth,
5,
Imaging Rheumatology bv,
4;
R. Landewé,
Abbott, Amgen, Centocor, Pfizer/Wyeth, UCB, and BMS,
2,
Abbott, Amgen, Centocor, Pfizer/Wyeth, UCB, and BMS,
5,
Abbott, Amgen, Centocor, Pfizer/Wyeth, UCB, and BMS,
8;
E. Keystone,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Genzyme, Merck, Novartis, Pfizer, Roche, and UCB,
2,
Abbott, AstraZeneca, Biotest, BMS, Centocor, Genentech, Merck, Nycomed, Pfizer, Roche, and UCB,
5,
Abbott, Amgen, BMS, Janssen, Merck, Pfizer, Roche, and UCB,
8;
F. C. Breedveld,
Centocor, Schering-Plough, Amgen/Wyeth, and Abbott,
5;
S. Liu,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
N. Mozaffarian,
Abbott Laboratories,
1,
Abbott Laboratories,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-integrated-radiographic-data-for-two-long-term-open-label-extension-studies-of-adalimumab/
