Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Some cases of progressive multifocal leukoencephalopathy (PML) have been described in patients with autoimmune diseases with profound immunosuppression, due to replication of JC polyomavirus within the CNS. Biologics such as natalizumab and efalzimab have been clearly associated with an increased risk of PML. Rare cases of PML have also been described in patients treated with rituximab (RTX), without any idea of the mechanisms which could support this association. Our objectives were to analyze JC viral load and anti-JC T-cell response in rheumatoid arthritis (RA) patients treated with RTX or anti-TNF.
Methods:
In four French rheumatology departments, RA patients starting a new biological therapy with either RTX or anti-TNF were recruited to participate in this prospective study. All patients were screened at baseline, 3 to 6 months and 12 to 18 months after the beginning of biologic therapy during for JC tests, including urine, PBMC and plasma viral load (with quantitative PCR method) and specific IFN-g Elispot for JC (Elipsot was performed after overnight activation of T cells with purified JCV). It was planned to include half of patients treated with RTX and half with anti-TNF, and to compare evolution of anti-JCV T cell response and JC viral load between these 2 groups.
Results:
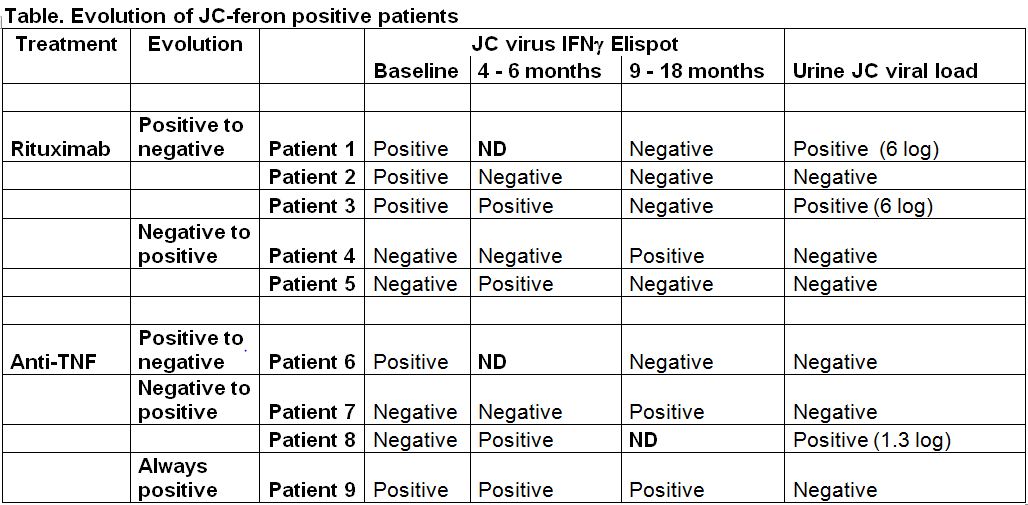
Between February 2010 and January 2011, 42 patients were included: 19 received RTX and 23 anti-TNF (etanercept n= 15; adalimumab n=3 ; infliximab n=4 ; golimumab n=1). At baseline and during the whole follow-up, all patients had negative PBMC and plasma viral load, 19/42 (45.2%) had positive and stable urine viral load. At baseline, 5 patients had positive JCV Elispot , 2 before anti-TNF and 3 before RTX, 2 out of these 5 patients having also positive urine viral load, one in each group. To assess reproducibility of the JCV Elispot , at baseline 4 patients had been tested twice a few days apart, results were similar (3 had negative and one had positive results).
38/42 patients had a follow-up visit. The evolution of JCV Elispot is reported the table. Overall, 9 patients had a positive JCV Elispot once, 5 at baseline, 2 after RTX and 2 after anti-TNF during the follow-up, including 3 who with positive urine viral load, which remained positive during the whole follow-up.
Conclusion:
The presence of anti-JCV T cell response has been found positive at least once in 9/42 (21%) of patients with RA treated with anti TNF or rituximab without any link with the use of one or the other type of biologics. These results suggest that in patients chronically infected with JC virus with long duration RA treated or not with biologics, transient reactivation of JC may occur leading to T-cell response Our data do not support a link with RTX or anti-TNF use. Long term follow up in larger cohorts of patients is necessary for indicating if a positive JCV Elispot could be predictive of further central neurological complication of JC virus.
Disclosure:
R. Seror,
Roche Pharmaceuticals,
5,
Pfizer Inc,
5,
Roche Pharmaceuticals,
2;
H. Chavez,
None;
A. A. Mazet,
None;
J. Sellam,
Roche Pharmaceuticals,
5,
Pfizer Inc,
5,
Abbott Laboratories,
5,
Schering-Plough,
5;
B. Fautrel,
Pfizer Inc,
9,
Roche Pharmaceuticals,
9,
Abbott Immunology Pharmaceuticals,
9,
Ucb,
9,
Roche Pharmaceuticals,
6,
Abbott Immunology Pharmaceuticals,
6,
Pfizer Inc,
6;
M. Dougados,
Pfizer Inc,
2,
Pfizer Inc,
6,
Pfizer Inc,
5,
Roche Pharmaceuticals,
2,
Roche Pharmaceuticals,
6,
Abbott Immunology Pharmaceuticals,
2,
Abbott Immunology Pharmaceuticals,
6,
UCB,
2,
Ucb,
6,
ucb,
5,
Roche Pharmaceuticals,
5,
Abbott Immunology Pharmaceuticals,
5;
Y. Taoufik,
None;
X. Mariette,
Pfizer Inc,
5,
UCB,
5,
Roche Pharmaceuticals,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-anti-jc-polyomavirus-t-cell-immune-response-with-jc-feron-in-patients-with-rheumatoid-arthritis-treated-with-rituximab-or-anti-tnf/