Session Information
Date: Sunday, November 17, 2024
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders I: Clinical Trials
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: A previous retrospective series reported the efficacy and tolerance of anakinra (ANK) in giant cell arteritis (GCA) and called for further validation in a prospective study.
Methods: This phase 3 study (NCT02902731) was a multicenter, randomized, double-blind, placebo-controlled trial over a 52-week period. Patients with a GCA diagnosis based on histology or large-vessel imaging were eligible. At inclusion, patients were randomized 1:1. From inclusion to week 16 (W16), patients in the anakinra (ANK) group received a daily 100mg injection of subcutaneous anakinra while patients in the placebo (PBO) group received a daily injection of placebo. In both arms, GC protocol was similar. Each patient started 0.7 mg/kg of prednisone equivalent and followed a protocolized tapering schedule until discontinuation at week 52. Endpoints included the relapse rate at W16, W26, W52, and the completion of the GC tapering. After determining the sample size, we aimed to include 70 patients, 35 in each arm. Given the SARS-CoV-2 pandemic, the study was prematurely stopped after 30 patients were included. We herein present the results of the intent-to-treat population of 30 patients.
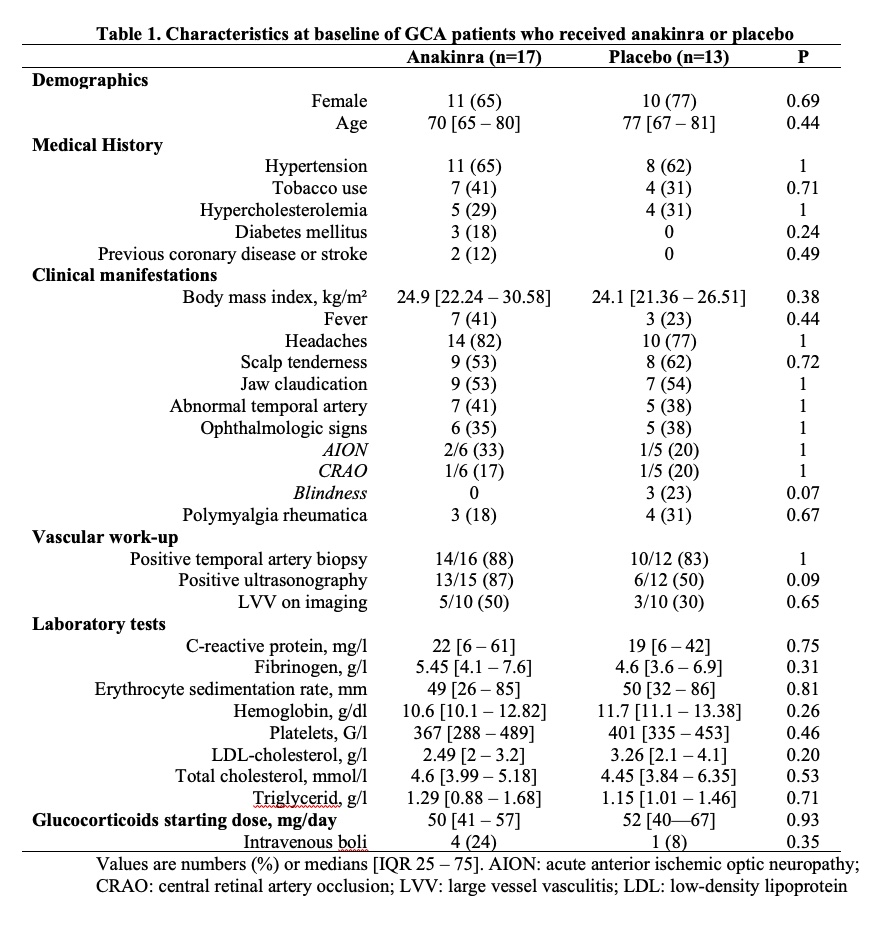
Results: From 5 centers, 30 patients were randomized as follows: 17 in the ANK group and 13 in the PBO group. Their characteristics at baseline were not different (Table 1). Of the 30 patients, 26 completed the W52 protocol visit. Among the 4 other patients, one in the ANK group was lost to follow-up at W42 and did not experience any relapse, two (one in each group) left the study protocol at W20 after experiencing relapses, and one in the ANK group stopped the study at W16 after he experienced two relapses.
Among the 17 patients who received ANK, one patient stopped the treatment at W12 after he experienced serious adverse events (AEs). He did not relapse before discontinuation.
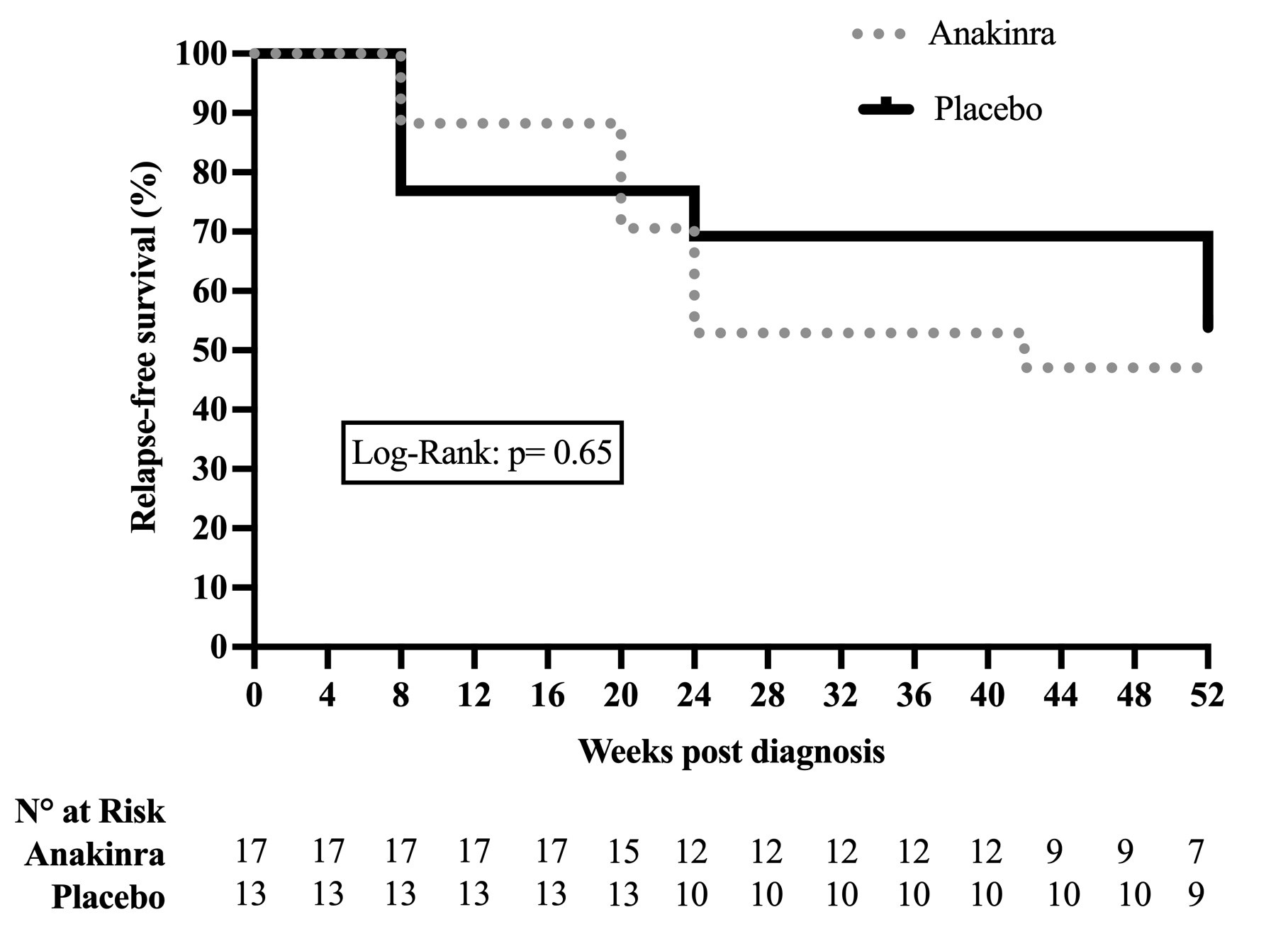
At W26, 12 (40%) patients had relapsed, 8 (47%) in the ANK group and 4 (31%) in the PBO group (p=0.47). No ischemic complication had occurred at the time of relapse. At W26, a deviation from the protocolized GC tapering was observed in 13 (43%) patients, 7 (41%) in the ANK group and 6 (46%) in the PBO group (p=1). At W52, a total of 15 (50%) patients had relapsed, 9 (53%) in the ANK group and 6 (46%) in the PBO group (p=1). The relapse-free survival is shown in Figure 1. Seven of the 9 relapsing patients who received ANK experienced a disease flare after ANK discontinuation. At W52, 2 patients in each group had discontinued GC (p=0.87). Seven serious AEs were reported in five patients, including 4 occurring under ANK. Infections were observed in 59% of patients who received ANK and 62% receiving PBO (p=1).
Conclusion: Although it was prematurely discontinued, this prospective study does not support anakinra as a treatment to reduce the risk of relapse in GCA or reduce GC exposure.
To cite this abstract in AMA style:
de Boysson H, Ly K, Geffray L, Quemeneur T, Liozon E, Bezanahary H, Le Gouellec N, Audemard A, Deshayes S, Boutemy J, Maigné G, Martin Silva N, Parienti J, Aouba A. Anakinra in Giant Cell Arteritis: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anakinra-in-giant-cell-arteritis-a-multicenter-randomized-double-blind-placebo-controlled-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anakinra-in-giant-cell-arteritis-a-multicenter-randomized-double-blind-placebo-controlled-trial/