Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Filgotinib (FIL) is a second-generation oral Janus kinase 1 preferential inhibitor approved in Europe, Japan, and the UK for treatment of RA.1,2 Over a median treatment duration of 2.2 years, similar incidence of adverse events of special interest (AESIs) were reported for FIL 200 mg (FIL200) and 100 mg (FIL100) dose groups, with the exception of infections, serious infections, and herpes zoster (HZ).3 This analysis provides an update on the safety profile of FIL.
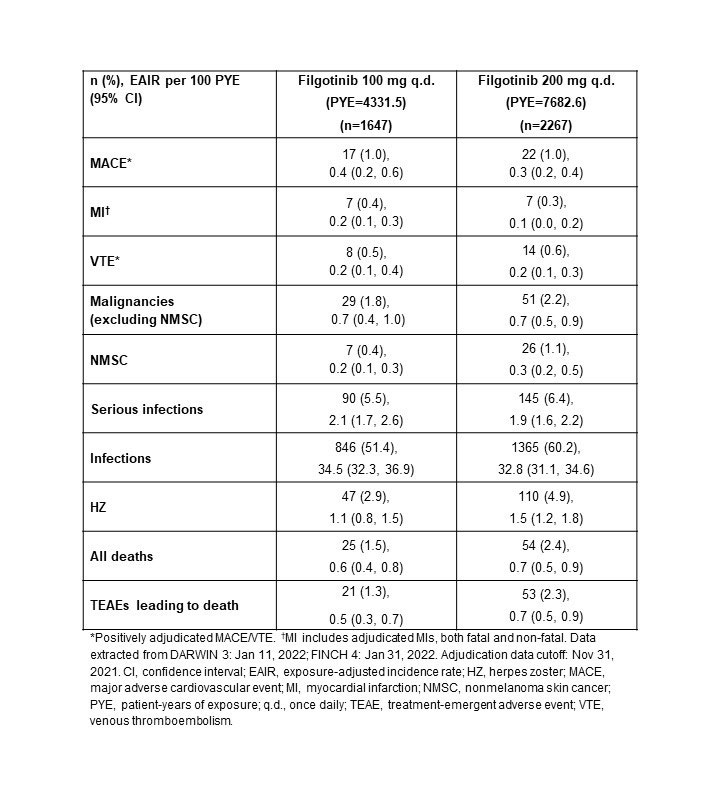
Methods: Integrated FIL RA data from 7 clinical trials are reported: phase 2 (NCT01668641, NCT01894516); phase 3 (NCT02889796, NCT02873936, NCT02886728); and the long-term extension studies, DARWIN 3 phase 2 (NCT02065700) and FINCH 4 phase 3 (NCT03025308). All patients met ACR criteria for functional class I–III. Exposure-adjusted incidence rates (EAIRs)/100 patient-years of exposure (PYE), censored at time of first event, were determined for major adverse cardiovascular event (MACE; including myocardial infarction), venous thromboembolism (VTE), malignancies excluding nonmelanoma skin cancer (NMSC), NMSC, (serious) infections, and deaths. Data were as of Jan 11, 2022 (DARWIN 3) and Jan 31, 2022 (FINCH 4). Analyses were performed on an ad hoc interim analysis data set without additional cleaning; MACE and VTE only include positively adjudicated events with a data cutoff of Nov 30, 2021.
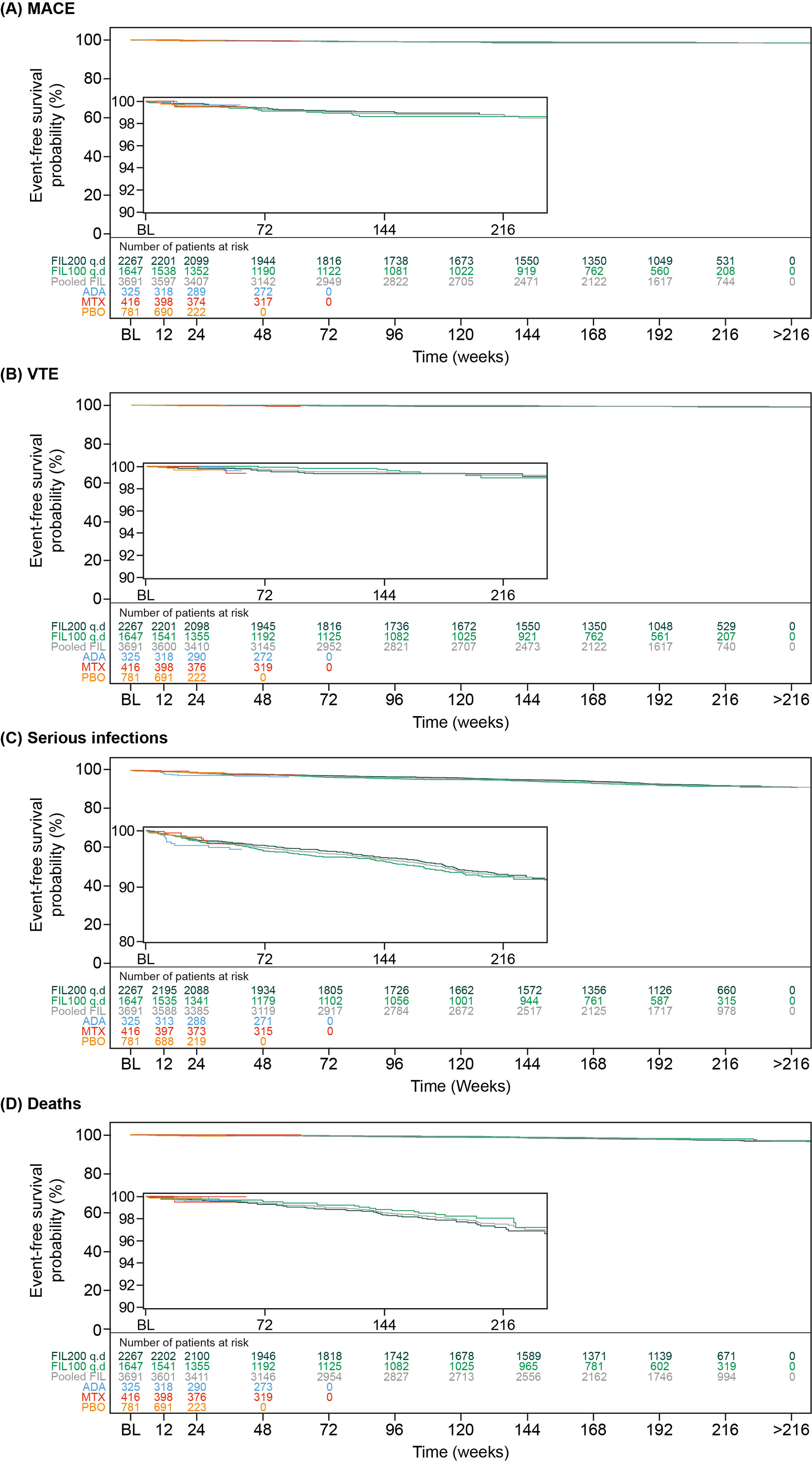
Results: Overall, the as-treated population included 3691 patients with 12,014.1 PYE. Median (max) exposure was 3.59 (8.2) years in the pooled FIL group, 3.69 (8.2) years for FIL200, and 3.15 (7.5) years for FIL100. Baseline demographics and disease characteristics were balanced between treatment groups.4 Small numerical differences were observed between FIL doses for EAIRs of AESIs, except for a higher incidence of HZ with FIL200 (Table). Of 22 treatment-emergent adverse event deaths associated with COVID-19, 1 patient had a record of vaccination. Risk of MACE and VTE was generally stable over time (Figure) with a probability of 1.4% vs 1.2% for FIL100 vs FIL200 for MACE at Week 216 (4 years). Risk of VTE was 0.7% and 0.6% at Week 192 for FIL200 and FIL100, respectively; at 216 weeks the probability of VTE was 1.0% vs 0.7% for FIL100 vs FIL200, although low event numbers make interpretation of any difference difficult. Over 216 weeks (4 years), the risk was generally similar between FIL100 vs FIL200 to develop malignancies excluding NMSC (3.1% vs 2.8%), serious infections (8.2% vs 7.7%) (Figure), or death from any cause (2.3% vs 2.8%) (Figure). The risk of experiencing NMSC was 0.9% vs 1.6% with FIL100 vs FIL200 at Week 216.
Conclusion: Over a median of 3.6 years, both FIL doses continue to show small numerical differences in EAIRs of AESI between FIL100 and FIL200 groups, except for HZ. In this overall RA population, FIL200 showed the highest risk for developing NMSC vs FIL 100, but not for MACE, malignancies excluding NMSC, serious infections, or death. Assessing risk between both doses for VTE was difficult due to low numbers.
References1. Jyseleca SmPC. Galapagos NV; May 2022
2. Jyseleca Japanese PI. Gilead Sciences K.K.; Sep 2020
3. Winthrop KL, et al. Arthritis Rheumatol 2021;73(S10):abstract 1698
4. Winthrop KL, et al. Ann Rheum Dis 2022;81:184–92
To cite this abstract in AMA style:
Winthrop K, Aletaha D, Caporali R, Tanaka Y, Takeuchi T, Van Hoek P, Watson C, Stiers P, Rajendran V, Van Beneden K, gottenberg j, Burmester G. An Update on the Integrated Safety Analysis of Filgotinib in Patients with Moderately to Severely Active RA [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/an-update-on-the-integrated-safety-analysis-of-filgotinib-in-patients-with-moderately-to-severely-active-ra/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-update-on-the-integrated-safety-analysis-of-filgotinib-in-patients-with-moderately-to-severely-active-ra/