Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteoarthritis Research Society International (OARSI) has launched an initiative to develop classification criteria for early-stage symptomatic knee osteoarthritis (EsSKOA). The goal is to establish a standardized way to identify and enrol individuals with symptomatic knee OA into clinical studies at an earlier stage of disease. To incorporate patient perspectives into the first phase (item generation), our objectives were to 1) develop a list of symptoms which, according to individuals living with knee OA, emerged within the first year of symptom onset; and 2) identify symptoms, signs and/or tests perceived to have helped them and their health professional(s) indicate their symptoms were due to knee OA and not another condition.
Methods: We conducted an international cross-sectional survey of people living with knee OA. We recruited participants from Australia, Canada, Netherlands, and USA. Included participants had to self-report a health-professional diagnosis of knee OA or be ≥45 years old, report activity-related knee pain, and indicate the ability to recall their earliest knee symptoms (within the first year from symptom onset). Participants completed an online questionnaire which asked if they experienced changes to how their knee looked, felt, moved, and overall wellbeing within the first year of knee symptom onset (5-point Likert from “definitely experienced” to “definitely did not experience”), and if experienced changes, describe specific symptoms using free-text. Participants were asked about specific symptoms, signs and/or tests that they perceived helped them and their health professional to know they had OA and not another condition. For quantitative data, we calculated the number and proportions or median and interquartile range, as appropriate. Analysis of free-text data was informed by conventional content analysis.
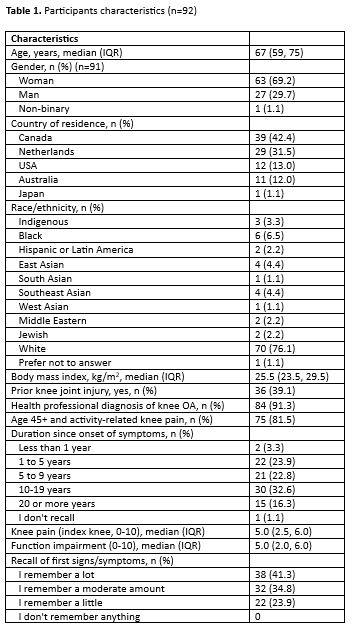
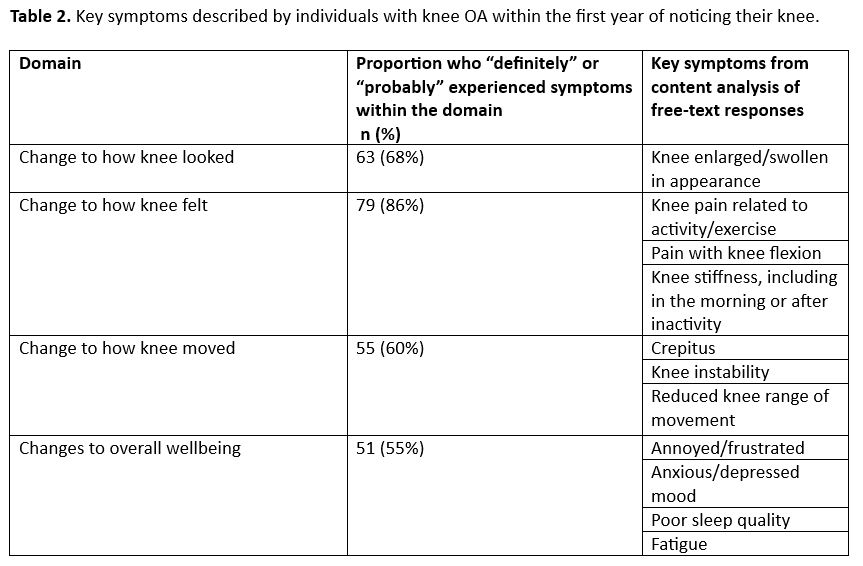
Results: We included 92 participants; mean age 68 years, 69% were women, 91% reported health-professional diagnosis of knee OA, and 27% reported onset of knee OA symptoms within the prior 5 years (Table 1). Within the first year of experiencing knee symptoms, the majority of participants “definitely” or “probably” experienced a change in how their knee looked (68%), felt (86%), moved (60%), or in overall wellbeing (55%). Specific symptoms within the first year described by >10% of participants are shown in Table 2. Only 35% of participants reported they sought care from a health professional within the first year of experiencing symptoms. Symptoms, signs and/or tests that participants indicated helped to determine they had knee OA and not another cause for knee symptoms, including clinical history (14%), physical exam (17%), and radiographs or other imaging (51%).
Conclusion: This international survey identified symptoms experienced by people living with knee OA within the first year of their illness. Given that few individuals seek healthcare within the first year, these provide key perspectives on the OA illness. The results of this study will be combined with those of a Delphi exercise with OA health professionals to generate a comprehensive list of items to be considered in subsequent phases of this initiative.
To cite this abstract in AMA style:
King L, Mahmoudian A, Liew J, Wang Q, Stanaitis I, Schiphof D, Callahan L, Hunter D, Appleton T, Turkiewicz A, Englund M, Lohmander S, Haugen I, Neogi T, Hawker G, Runhaar J. An International Patient Survey to Identify Candidate Items for Classifying Early-Stage Symptomatic Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/an-international-patient-survey-to-identify-candidate-items-for-classifying-early-stage-symptomatic-knee-osteoarthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-international-patient-survey-to-identify-candidate-items-for-classifying-early-stage-symptomatic-knee-osteoarthritis/