Session Information
Date: Monday, November 13, 2023
Title: (1013–1032) Healthcare Disparities in Rheumatology Poster II: Socioeconomic Determinants
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The randomized controlled trial (RCT remains a most important research tool and a necessary document for drug licensing. Informed consent is an integral component of this tool. Its exact wording is not currently in the public domain as we and others had previously voiced (1, 2). Such transparency is important not only from the point of maintaining the public trust but also for an organized skepticism, an essential element of scientific inquiry (3). Moreover, we had also indicated that this transparency was even more important in safety outcome trials (SOTs) (4). We have the impression that such SOTs are becoming more frequent and, in some, the outcome measure can be as severe as stroke or death. We aimed to tabulate the temporal frequency of RCTs with the primary outcome of primarily safety versus primarily efficacy, in 4 mainline general medicine journals.
Methods: RCTs published in 1990-1991 vs 2019-2020 in NEJM, JAMA, LANCET and BMJ were surveyed by 2 independent reviewers (AO, SNE). Phase 1-2 RCTs, post-hoc analyses of RCTs and RCTs reporting long term follow-up data were excluded. RCTs with an undisputable study aim and a primary endpoint of efficacy with no appreciable risk of harm to the patients were defined as an ‘A trial’. Those trials in which we unanimously considered the presence of serious harm to the enrolled patients were defined as a ‘B trial’. The trials that we could not unanimously decide whether to designate as an A or B, were called a ‘C trial’. Discrepancies were resolved after discussion with HY and if there was no consensus, the trial was designated as ‘non-A’. We compared the frequencies of the types of RCTs published in 1990-1991 vs 2019-2020. Other salient features of these RCTs were also tabulated. Of these, only data related to the intensive care unit (ICU) settings are presented in this abstract.
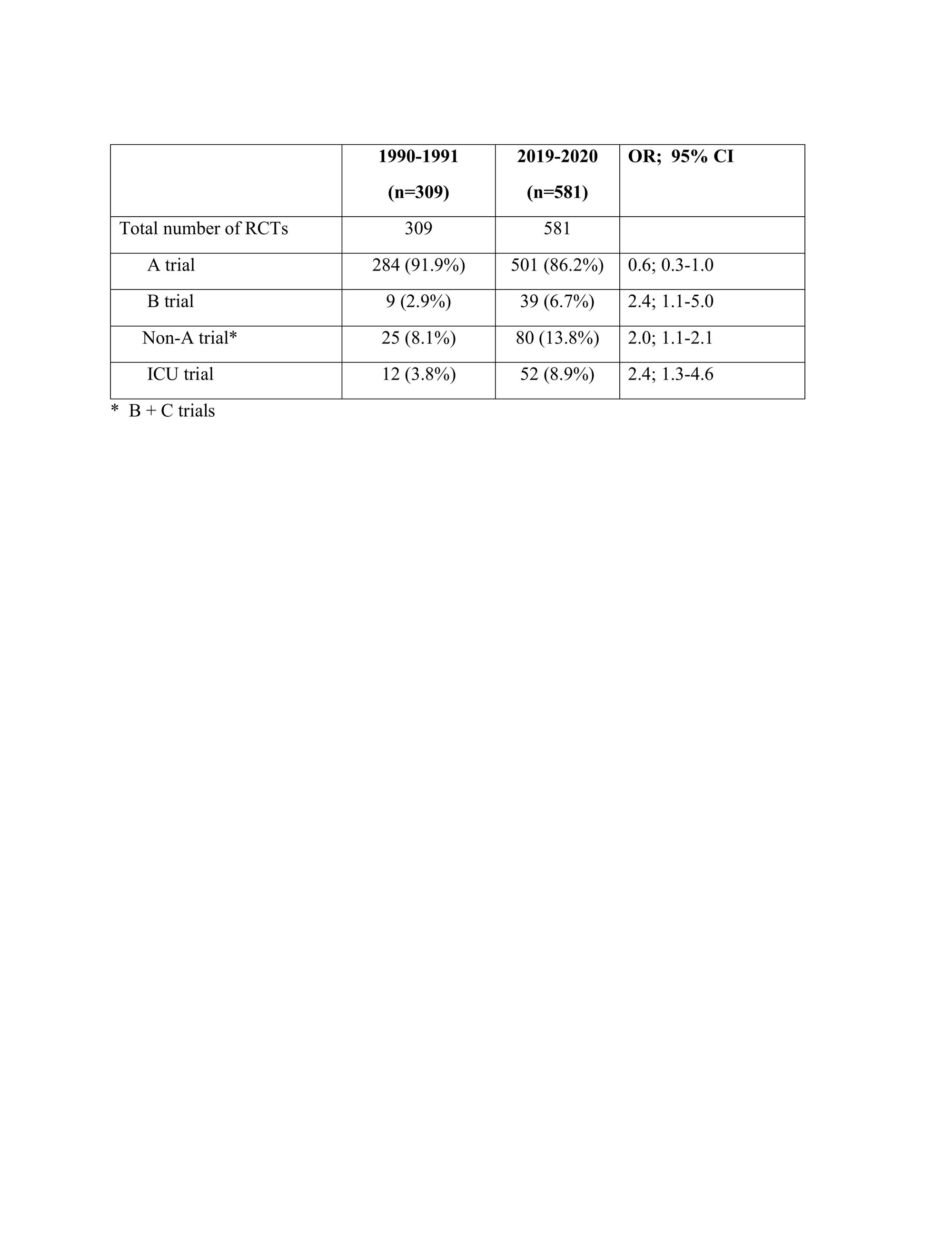
Results: There were 309 RCTs published in 1990-1991 and 600 RCTs published in 2019-2020. 19 RCTs on COVID-19 infection were excluded for fair comparisons. As seen in the Table there was a significant increase in the number SOTs in the later years. Moreover, the number of ICU trials, a setting in which obtaining informed consents can often be problematic, evento the degree of being post-hoc, were significantly increased.
Conclusion: There is a significant increase in the number of SOTs. Moreover, more RCTs are conducted in the ICUs. We re-emphasize that the informed consents of all RCTs, particularly those related to the SOTs should be in the public domain. We strongly consider that such action is necessary a. to continue receiving the necessary public trust for scientific inquiry and b. for adequately addressing the organized skepticism of our peers.
References
1. Yazici Y, Yazici H. Informed consent: time for more transparency. Arthritis Res Ther 2010;12: 121.
2. Kotz D, et al. Details about informed consent procedures of RCTs should be reported transparently. J Clin Epidemiol 2019;109: 133-5.
3. Merton, et al. Science and technology in a democratic order. J Legal and Political Sociology 1942; 1:115–26.
4. Ozdede A, Yazıcı H. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. NEJM 2022; 386:1766.
To cite this abstract in AMA style:
Ozdede A, Esatoglu S, Yazici H. An Increase in Safety Outcome Trials and the Issue of Informed Consent [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/an-increase-in-safety-outcome-trials-and-the-issue-of-informed-consent/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-increase-in-safety-outcome-trials-and-the-issue-of-informed-consent/