Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Spondyloarthritis Including PsA – Treatment I: Axial Spondyloarthritis
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: In clinical trials of axial spondyloarthritis (axSpA), composite measures such as Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) response criteria, Assessment of Spondyloarthritis international Society (ASAS) response criteria, Ankylosing Spondylitis Disease Activity Score (ASDAS) disease activity states and ASDAS response criteria are used. However, these validated measures are largely based on patient (pt)-reported symptoms, which are likely to be confounded by other factors. In pts with active axSpA, certolizumab pegol (CZP) has demonstrated sustained clinical efficacy in clinical outcomes over 204 weeks.1 However, we hypothesize that reduction of sacroiliac joint (SIJ) and spinal inflammation following treatment with CZP, as measured by MRI and CRP levels,2 may not necessarily translate into clinical response. This post hoc analysis aims to evaluate the relationship between objective signs of inflammation (OSI), as measured by MRI/CRP, and clinical outcomes following 12 weeks of CZP treatment in pts with active axSpA.
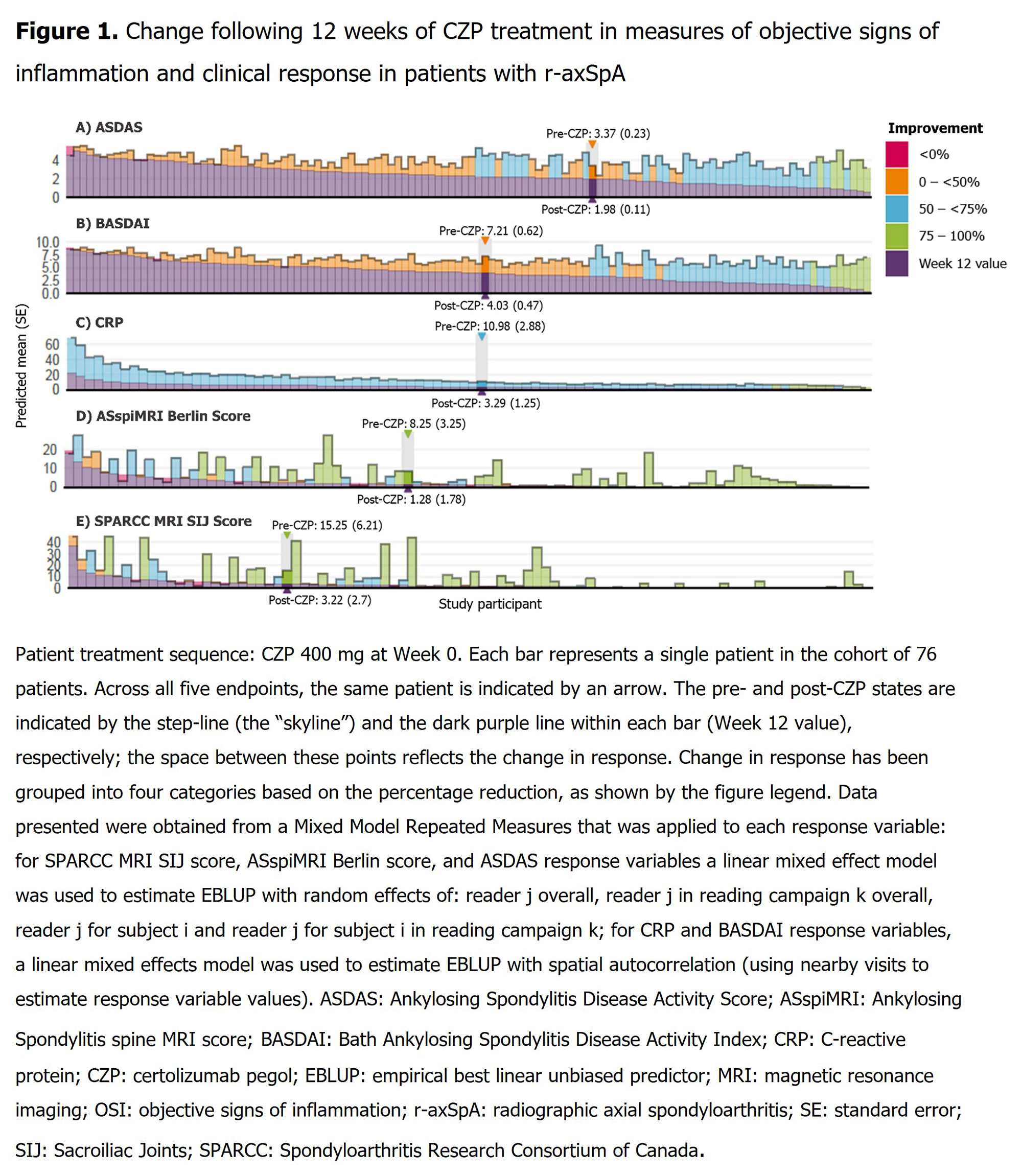
Methods: Data were analyzed from the phase 3 RAPID-axSpA study (NCT01087762).1 To improve precision of pt-level estimates, data from two independent readers were pooled in a Mixed Model for Repeated Measures for each response variable; Manhattan plots are presented for ASDAS, BASDAI, CRP, Ankylosing Spondylitis spine MRI (ASspiMRI) Berlin Score and MRI Spondyloarthritis Research Consortium of Canada (SPARCC) SIJ score. Outcomes are reported for pts (initially randomized to either CZP, or to placebo [PBO] before switching to CZP) prior to CZP treatment (pre-CZP treatment) and following 12 weeks (wks) of treatment (post-CZP treatment).
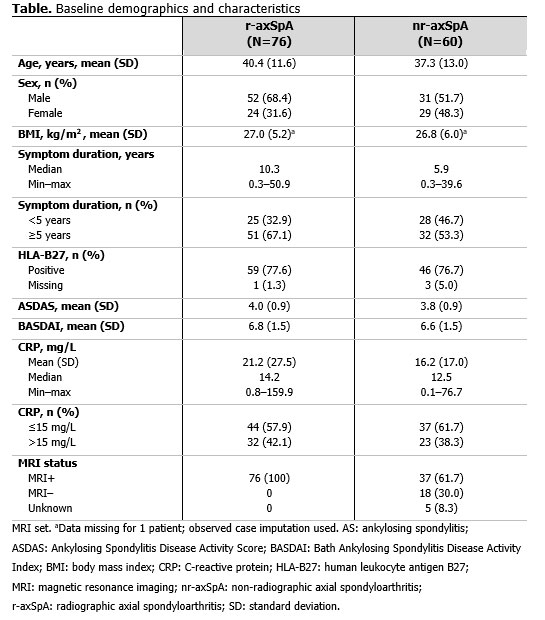
Results: 136 pts (radiographic axial spondyloarthritis [r-axSpA]: 76; non-radiographic axial spondyloarthritis [nr-axSpA]: 60) had a pre-CZP and ≥1 post-CZP MRI. Pre-CZP demographics for the r-axSpA and nr-axSpA populations are presented in Table. Following CZP treatment, CRP, ASspiMRI Berlin score, and MRI SPARCC SIJ score were reduced by >50%, in the majority of pts (CRP: 136/136 [100.0%]; Berlin: 73/136 [53.7%]; SPARCC: 71/136 [52.2%]), and often by >75% (CRP: 18/136 [13.2%]; BERLIN: 53/136 [39.0%]; SPARCC: 50/136 [36.8%]) irrespective of pre-CZP value. However, only a minority of both r-axSpA (Figure 1) and nr-axSpA pts (Figure 2), showed a >50% reduction in clinical responses (BASDAI: 64/136 [47.1%]; ASDAS: 66/136 [48.5%]). These results were also observed at the individual pt level; >50% improvements in MRI/CRP inflammatory measures did not translate into similar improvements in clinical responses for the majority of pts.
Conclusion: This post hoc analysis reveals a potential disconnect between OSI, as measured by MRI and CRP, and clinical outcomes responses. In the majority of pts, CZP treatment resulted in a reduction of objective measures of inflammation, irrespective of improvements in clinical symptoms and measures of disease activity. The use of clinical response measures as clinical trial endpoints may therefore underestimate anti-inflammatory treatment effects.
References: 1. van der Heijde. Ann Rheum Dis 2017;77:699–705; 2. Braun J. RMD Open 2017;3:e000430.
To cite this abstract in AMA style:
Rudwaleit M, Van den bosch F, Marzo-Ortega H, Navarro-Compán V, Tham R, Kumke T, Bauer L, Kim M, Gensler L. An Exploratory Analysis of the Potential Disconnect Between Objective Inflammatory Response and Clinical Response Following Certolizumab Pegol Treatment in Patients with Active Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/an-exploratory-analysis-of-the-potential-disconnect-between-objective-inflammatory-response-and-clinical-response-following-certolizumab-pegol-treatment-in-patients-with-active-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-exploratory-analysis-of-the-potential-disconnect-between-objective-inflammatory-response-and-clinical-response-following-certolizumab-pegol-treatment-in-patients-with-active-axial-spondyloarthritis/