Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The autoinflammatory diseases are diseases in which there is seemingly unprovoked stimulation of the innate immune system. The enhanced release of cytokines in these diseases (TNF-α, IL-1, IL-6), enable biologic medications targeting these cytokines to be good treatment options. The IL-1 antagonist, anakinra, has been approved for use in rheumatoid arthritis and neonatal onset multisystem inflammatory disease (NOMID) but is also frequently used in other autoinflammatory diseases such as the tumor necrosis factor associated periodic syndrome (TRAPS), familial Mediterranean fever (FMF), familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome. In addition, anakinra is often used in patients with undiagnosed autoinflammatory syndromes. Although commonly effective at the approved daily dose of 100mg, there are a number of patients who continue to have frequent disease flares and documented inflammation. We have found that aggressive titration of anakinra can help to better control disease and that higher doses have not been found to have increased adverse events.
Methods: A retrospective chart review of adult patients seen in the National Institutes of Health Autoinflammatory Disease clinic was completed. Patients on daily anakinra were assessed for what dose of anakinra provided adequate disease control. Number and types of adverse events were noted.
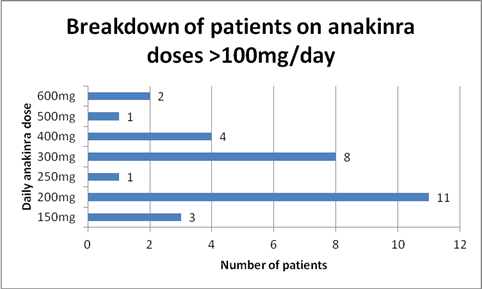
Results: 56 patients were identified who were taking at least 100mg daily of anakinra. 26 patients were on 100mg daily. The daily dose of the remaining 30 patients is detailed in Figure 1. Comparing patients on 100mg daily with those on higher doses, local site reactions were the most common adverse event in both groups (NS). Regarding more serious adverse events, in the 100mg group there was 1 patient who developed viral meningitis and one who developed a facial abscess. In patients on greater than 100mg daily, one patient with a chronic PICC line developed Serratia bacteremia and one patient with PAPA syndrome developed a C. difficile infection after treatment with clindamycin for presumed skin infection. Inflammation was able to be suppressed in 24/26 patients (92%) and 3/3 patients who underwent organ transplantation secondary to AA amyloidosis (2 kidney and 1 liver) had viable transplants at 7, 9, and 16 years post-transplant on 300, 600, and 200mg anakinra respectively.
Conclusion: In autoinflammatory disease patients who have inadequate disease suppression on anakinra 100mg daily, an upward titration of anakinra is relatively safe and well-tolerated. Close physician supervision is needed to try and minimize adverse events. Local site reactions are the most commonly reported adverse event and the majority of patients have resolution of these reactions within one month of initiating therapy.
Disclosure:
A. K. Ombrello,
None;
K. Barron,
None;
P. M. Hoffmann,
None;
A. Jones,
None;
D. Stone,
None;
D. L. Kastner,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-escalating-dose-of-anakinra-in-patients-with-autoinflammatory-disease-is-a-safe-and-reasonable-therapeutic-option/