Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose:
Diagnosis (dx) of systemic lupus erythematosus (SLE) is made via a combination of clinical and laboratory examinations; the sensitivity and specificity (S&S) of standard diagnostic tests (SDTs) (i.e.: ANA and antibodies to dsDNA, Smith, Ro/SSA, La/SSB, centromere, Jo-1, Scl-70, and CCP), are reported to be 83% & 76%, respectively (Putterman et al, 2014). A multivariate assay panel (MAP) combining biomarkers, complement C4d activation products on erythrocytes & B cells, with SDTs, yields improved dx performance with a S&S of 80% & 86%, respectively (Putterman et al, 2014). We evaluated the payer budget impact of SLE dx using MAP compared to SDTs.
Methods:
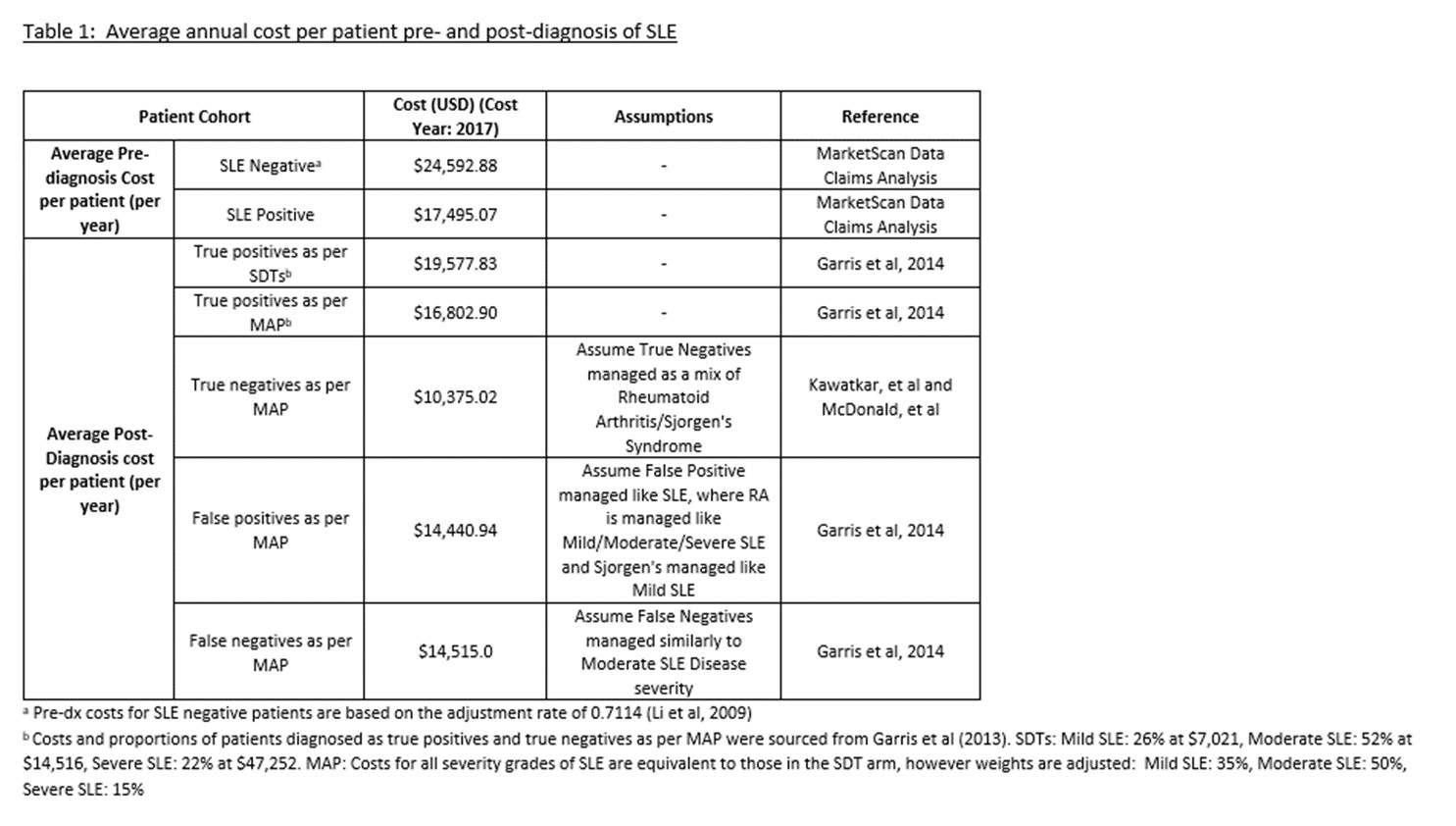
We modeled a health plan of 1 million enrollees, with 0.1% suspected of SLE. SLE dx among suspected SLE patients was 9.2% (Djikstra et al, 1999). The MAP arm assumed 80% /20% of suspected SLE patients were tested with MAP/SDTs, compared to 100% SDT testing in SDT arm. Based on improved MAP performance, the hazard ratio for the assumed dx rate compared to SDTs was 1.74, (71, 87, 90, and 91% of the suspected who develop SLE will be diagnosed in years 1 – 4 compared to 53, 75, 84, and 88% with SDTs). Increased MAP performance relative to SDTs should result in earlier dx of SLE, reduction in disease severity at dx, and a longer period of time in less severe disease states (Oglesby et al, 2014) with associated lower costs. Pre-SLE dx claims-based cost data was used to estimate recurring direct costs for undiagnosed patients, and a weighted average of post-dx direct cost data for mild, moderate, and severe SLE were obtained from the literature (Garris, 2014) for SDTs, which was re-weighted to reflect the MAP scenario (Table 1).
Results:
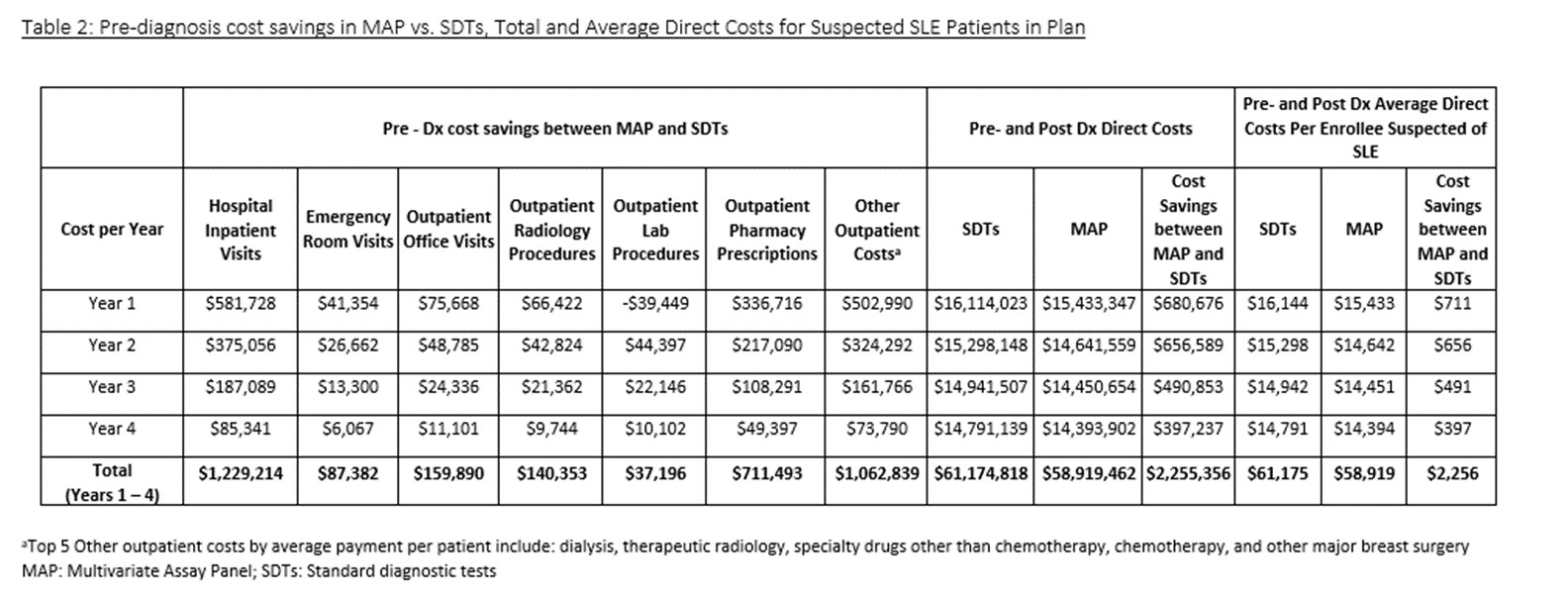
Total 4-year pre- and post- dx direct medical cost for suspected SLE patients tested with MAP were $58,919,462 compared to $61,174,818 by SDTs (Table 2). Total 4-year average cost savings per suspected SLE patient tested with MAP were $2,256 (Table 2). Reduced inpatient hospital admissions were the biggest driver of cost savings ($581,728 in yr 1). In a one-way sensitivity analysis, the percentage of SLE dx among those suspected of SLE and specificity of MAP had the largest impact on average annual cost savings (respective savings of $16 and $15 per 1% absolute increase in percentage of SLE among those suspected of SLE and MAP specificity).
Conclusion:
Incorporating MAP into SLE dx results in total 4-year direct cost savings of $2,255,356 ($2,256 per suspected SLE patient) and year 1 savings of $711 per suspected SLE patient. Further, by facilitating earlier dx of SLE at a less severe disease state, it is anticipated that MAP will enhance patient outcomes.
Tables and Figures:
To cite this abstract in AMA style:
Clarke AE, Goss T, Piscitello A, Chandra T, O'Malley T, Dervieux T, Powell T. An Economic Evaluation on the Impact of Earlier Systemic Lupus Erythematosus (SLE) Diagnosis Using Complement C4d Activation Products in a Multivariate Assay Panel (MAP) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/an-economic-evaluation-on-the-impact-of-earlier-systemic-lupus-erythematosus-sle-diagnosis-using-complement-c4d-activation-products-in-a-multivariate-assay-panel-map/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-economic-evaluation-on-the-impact-of-earlier-systemic-lupus-erythematosus-sle-diagnosis-using-complement-c4d-activation-products-in-a-multivariate-assay-panel-map/