Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Inflammatory stimuli induce transcription factors (TFs) such as NF-kB and interferon regulatory factors (IRFs). TFs are transported from cytosol to nucleus to activate genes encoding chemokines and cytokines. Nuclear transport of TFs is performed by importins that comprise a beta chain and different alpha chains that confer substrate specificity for TF nuclear localization sequences (NLS). The alpha-5-containing importin (Impα5) is of particular interest because it transports STAT1, NF-kB, and other stress responsive TFs containing a NLS. AMTX-100 is a 28 amino acid chimeric peptide containing a huFGF4 domain and NF-kB p50 NLS that function to facilitate leukocyte cell penetration and as a nuclear transport checkpoint inhibitor (NTCI), respectively. AMTX-100 is currently in a Phase 2b clinical trial to treat inflammation in eczema. Since Impα5 binds to the NLS of NF-kB p50 as well as STAT1 & 3, we examined the effects of AMTX-100 on inflammatory pathways and Type I interferons (IFN-I), in vitro and in vivo.
Methods: The human monocyte cell line, THP-1 was transfected with 0.1 ug dsDNA in the presence or absence of AMTX-100 and cytokine mRNA expression quantified by qPCR. Neutrophils from healthy volunteers were isolated by density gradient and exposed to either PMA, the ionophore A23187 or immune complexes (IC) containing the lupus antigen SmRNP. NET formation was quantified by release of DNA and MPO as determined by fluorimetry. Female C57BL/6 (B6) mice aged 8-12 weeks were exposed to a single dose of UV (500mJ/cm2) on the dorsal skin. Skin biopsies were obtained before and at 6 and 24 hr post UV exposure. Cytokine gene expression was determined by qPCR. Statistical significance between groups was determined by Student’s t-test.
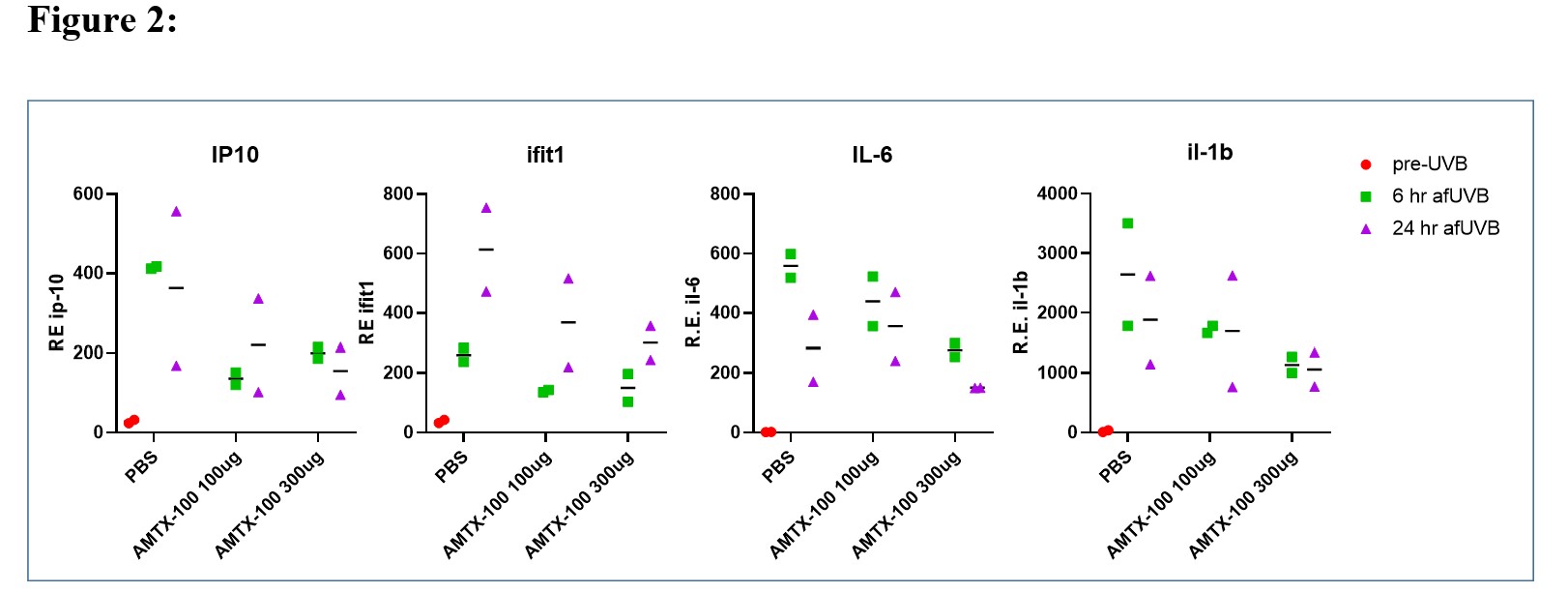
Results: In vitro studies with THP-1 cells revealed that 1 uM AMTX-100 inhibited IFN-b expression by 82% , and IL-6, TNF and IL-1b by 98%, 84%,and 95% respectively (Fig. 1). In neutrophils incubated with NET-inducing stimuli, AMTX-100 (3 uM) inhibited DNA and MPO release by ~40% in PMA or A23187 stimulated cells but did not affect NET release induced by IC. To determine whether AMTX-100 attenuated the IFN-I response following UV induced inflammation of mouse skin, we applied either 100 or 300 ug AMTX-100 in saline onto the skin before and once daily after UV exposure. As shown in Fig. 2, ISGs, as well IL-1 and IL6 were all reduced in an apparent dose dependent fashion. Similar results were obtained in a second in vivo experiment (not shown).
Conclusion: We conclude that AMTX-100 reduces Type I IFN and NF-kB-dependent inflammatory cytokines from DNA activated human monocytes in vitro. While AMTX-100 had a modest inhibitory effect on NET formation in response to PMA and ionophore stimulation, it did not attenuate NET formation induced by ICs in vitro. When tested in vivo using the lupus-relevant UV skin exposure, we observed a dose-dependent inhibition of several NF-kB-dependent cytokines as well as ISGs. Since AMTX-100 is in clinical trials for inflammatory skin disease, these findings suggest a novel therapeutic approach utilizing importin NTCIs that may be useful for prevention or treatment of cutaneous lupus erythematosus.
To cite this abstract in AMA style:
Sun X, An J, Wang T, Rathee A, Alvarez V, Gonda M, Lood C, Elkon K. AMTX-100, a Nuclear Transport Inhibitor, Attenuates Inflammatory Cytokine Production in vitro and Following UV Mediated Skin Inflammation in a Mouse Model of Cutaneous Lupus Erythematosus in vivo [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/amtx-100-a-nuclear-transport-inhibitor-attenuates-inflammatory-cytokine-production-in-vitro-and-following-uv-mediated-skin-inflammation-in-a-mouse-model-of-cutaneous-lupus-erythematosus-in-vivo/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/amtx-100-a-nuclear-transport-inhibitor-attenuates-inflammatory-cytokine-production-in-vitro-and-following-uv-mediated-skin-inflammation-in-a-mouse-model-of-cutaneous-lupus-erythematosus-in-vivo/