Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Belimumab (BEL), approved in active, autoantibody-positive SLE, has demonstrated a positive efficacy/safety profile while suggesting potential for steroid sparing and reduced organ damage accrual. BASE, a placebo-controlled study, evaluated all-cause mortality and adverse events of special interest (AESIs), and limited efficacy endpoints.
Methods: Adults who met ACR SLE criteria were randomized (1:1) to monthly BEL 10 mg/kg IV or placebo (PBO) plus standard of care for 48 weeks. There was no minimum disease activity required or exclusions for psychiatric conditions, and no protocol mandated corticosteroid (CS) taper or assessment of change in disease activity. For primary endpoints of mortality and AESIs (reported previously), differences in rates (95% CI) were assessed vs PBO on-treatment (first to last dose +28 days). Serious suicidal ideation/behavior and self-injury events on-treatment, and C-SSRS suicidal ideation/behavior on-study (first dose to end of Week 52 follow up) were assessed (differences calculated post hoc). Percentages of patients with baseline CS dose >7.5 mg/day whose dose to treat SLE reduced by ≥25% to ≤7.5 mg/day (Weeks 40‒52), SLE immunomodulator use (baseline vs treatment completion), study hospitalizations, and organ damage accrual (Week 52), were assessed.
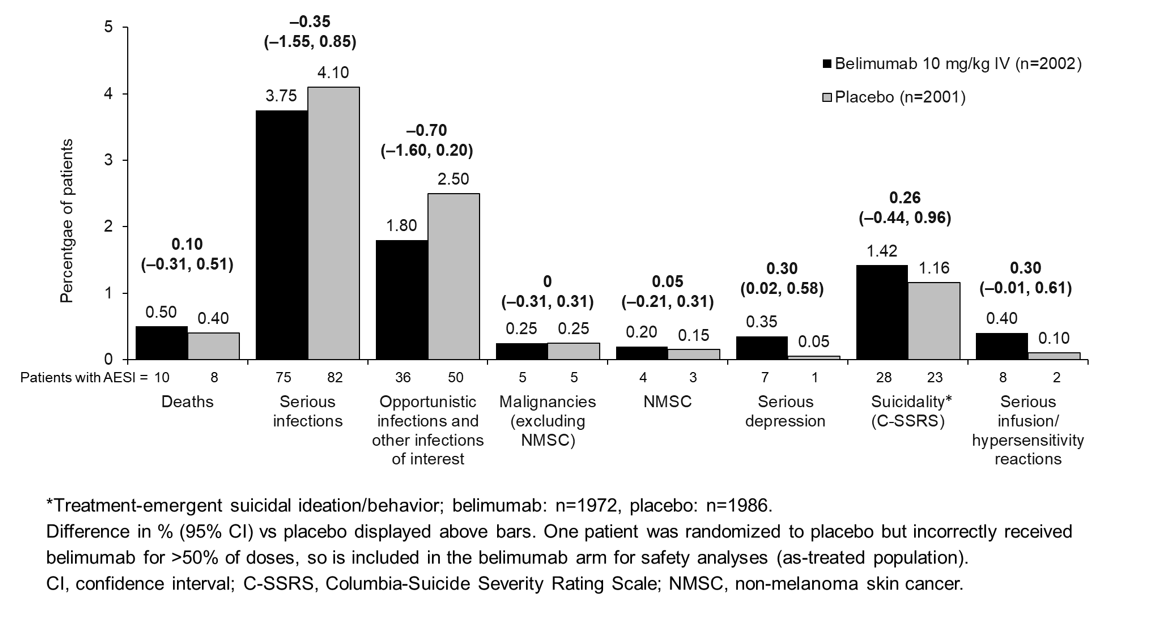
Results: Baseline demographics and disease activity/characteristics were similar between groups. All-cause mortality was also similar (Figure); most deaths were due to infections (9 [0.45%] BEL, 3 [0.15%] PBO). On-study deaths occurred in 13 (0.65%) BEL and 22 (1.10%) PBO patients (difference [95% CI]: –0.45 [–1.03, 0.13]). Rates of on-treatment AESIs were similar, except for serious depression and serious infusion/hypersensitivity reactions (Figure).
On-treatment serious suicidal ideation/behavior and self-injury sponsor-adjudicated events occurred in 15 (0.75%) BEL and 5 (0.25%) PBO patients (difference [95% CI]: 0.50 [0.06, 0.94]); on-study suicidal ideation/behavior (C-SSRS) occurred in 48 (2.43%) BEL and 39 (1.96%) PBO patients (difference [95% CI]: 0.47 [–0.44, 1.38]). There were no suicide-related deaths.
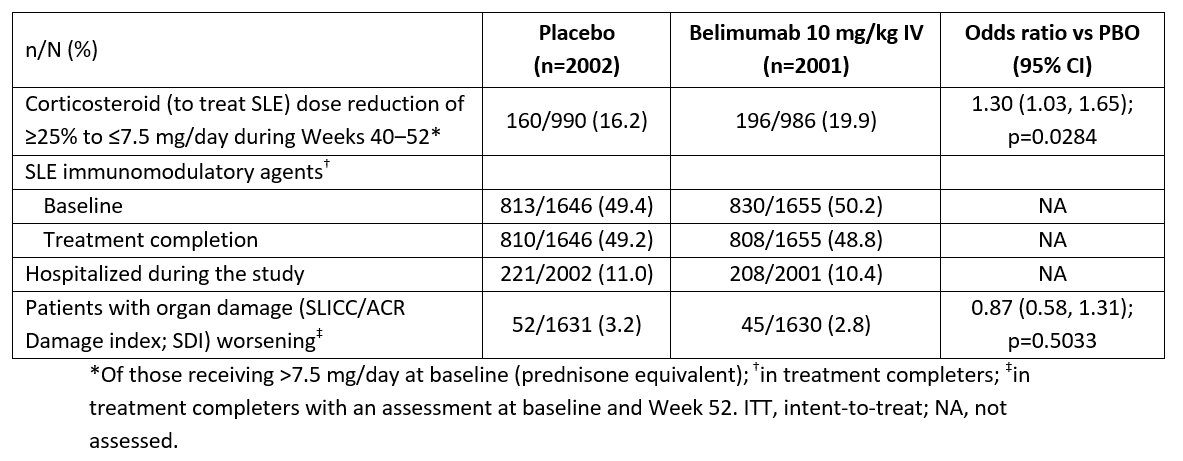
Efficacy endpoints were assessed in those receiving ≥1 dose (intent-to-treat; 2001 BEL, 2002 PBO). More BEL vs PBO patients had a SLE CS dose reduction (Table). SLE immunomodulator use did not change vs baseline. Hospitalizations were similar between groups. BEL impact on organ damage, if any, was minimal.
Conclusion: In the largest, double-blind, placebo-controlled SLE study to date, on-treatment all-cause mortality, infection, and malignancy AESI rates were similar between BEL and PBO; imbalances were observed in serious depression, serious suicidal ideation/behavior and self-injury events, and serious infusion/hypersensitivity reactions. BEL reduced SLE CS use in high-dose patients; the effect was small and without supporting parallel disease activity measures. There was no change in SLE immunomodulator use, no differences in hospitalizations, and organ damage impact was minimal, as in other BEL studies.
We acknowledge the BASE participants and Study Group. Study funding: GSK. Medical writing support: Louisa McKay, PhD, Fishawack Indicia Ltd, UK (funded by GSK).
To cite this abstract in AMA style:
Sheikh S, Scheinberg M, Wei C, Tegzova D, Stohl W, Acayaba de Toledo R, Mucenic T, Abello Banfi M, Maksimowicz-McKinnon K, Abud-Mendoza C, Navarra S, Garcia M, Garcia-De La Torre I, Ordi Ros J, Levy R, Bass D, Ross Terrés J, Punwaney R, Harris J, Nami A, Pierce A, Thorneloe K, Ji B, Roth D. Adverse Events of Special Interest, SLE Medication Utilization, Hospitalizations, and Organ Damage: Results from a Phase 4, Randomized, Double-Blind, Placebo-Controlled, 52-week Study of Belimumab in Adults with Active, Autoantibody-Positive SLE [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/adverse-events-of-special-interest-sle-medication-utilization-hospitalizations-and-organ-damage-results-from-a-phase-4-randomized-double-blind-placebo-controlled-52-week-study-of-belimumab-in/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adverse-events-of-special-interest-sle-medication-utilization-hospitalizations-and-organ-damage-results-from-a-phase-4-randomized-double-blind-placebo-controlled-52-week-study-of-belimumab-in/