Session Information
Date: Sunday, November 13, 2022
Title: Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: FDA approved belimumab, the first targeted biological treatment for SLE, in March 2011 for adults with active, seropositive SLE receiving standard therapy. The indicated population for belimumab for SLE was expanded in April 2019 to include pediatric patients 5-17 years old, making belimumab the first approved treatment for pediatric patients with SLE. FDA approval relied on a randomized clinical trial (RCT) conducted in pediatric patients that evaluated the efficacy, safety, and pharmacokinetics of belimumab. However, the rarity of childhood-onset SLE limited RCT enrollment; therefore, efficacy was also supported by extrapolation of efficacy from adult studies. FDA conducts ongoing postmarketing safety surveillance, including the monitoring of adverse event (AE) reports submitted to the FDA Adverse Event Reporting System (FAERS) database. The purpose of this study is to provide a descriptive analysis of the postmarketing AE reports in FAERS for belimumab use in pediatric patients.
Methods: We searched FAERS for all AEs reported with belimumab for pediatric patients, age 0-17 years old, from March 9, 2011 (approval date of belimumab) through March 22, 2022. We excluded reports that 1) lacked sufficient information for case assessment, 2) described transplacental exposure, 3) did not describe AEs with belimumab, 4) were derived from a previously reviewed clinical trial, 5) were duplicative, 6) had miscoded age.
Results: We identified 27 FAERS cases reporting AEs in pediatric patients with belimumab, including 8 cases (30%) received from the U.S. and 19 (70%) from foreign countries. Fifteen (55%) were reported by physicians, 8 (30%) reported by other health care professionals and 4 (15%) reported by consumers. The mean reported age was 15 years (range 4 to 17 years). The cases reported 20 (74%) females and 7 (26%) males. The reported indication for use was SLE for 23 (85%) cases and complement deficiency disease for 1 (4%) case; 3 (11%) cases did not report an indication for use. Eleven (41%) cases reported hospitalization, and 1 (4%) case reported fatality due to a pulmonary infection complicated by sepsis.
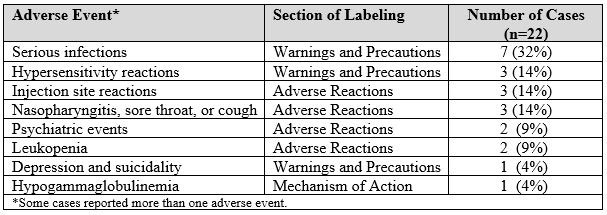
Of the 27 FAERS cases associated with belimumab, 22 (81%) cases reported AEs that are listed in the current belimumab labeling, and 5 (19%) cases reported unlabeled AEs. Some cases reported more than one AE. Table 1 lists the labeled AEs and Table 2 lists the unlabeled AEs reported with belimumab in the FAERS database. The case narratives had limited information to determine whether the unlabeled AEs were related to belimumab exposure.
Conclusion: Evaluation of the available FAERS postmarketing data did not identify new safety concerns for belimumab use in pediatric patients. Most reported AEs are consistent with known AEs described in the current belimumab labeling. Few pediatric AEs have been reported to FAERS since approval, and healthcare providers are strongly encouraged to report AEs. Continued pharmacovigilance is important to understand the full spectrum of belimumab safety. Further evaluation using all available data sources is needed before further conclusions about belimumab safety can be made.
To cite this abstract in AMA style:
Kim I, Ryan D, Cheng C, Kortepeter C. Adverse Events Associated with Belimumab Use in Pediatric Patients: Review and Analyses of the FDA Adverse Event Reporting System Database [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/adverse-events-associated-with-belimumab-use-in-pediatric-patients-review-and-analyses-of-the-fda-adverse-event-reporting-system-database/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adverse-events-associated-with-belimumab-use-in-pediatric-patients-review-and-analyses-of-the-fda-adverse-event-reporting-system-database/