Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Sodium glucose cotransporter 2 inhibitors (SGLT2i) are oral hypoglycemic agents for Type II diabetes mellitus (T2D) now prescribed for renal and cardiovascular indications. Patients with autoimmune rheumatic diseases (ARD) have been excluded from SGLT2i clinical trials due to theoretical increased infection risk in the immunosuppressed. We compared adverse events associated with SGLT2i prescription in patients with vs. without ARD.
Methods: Using a large data repository, we identified patients with ≥2 ICD10 diagnostic codes for ARD who were prescribed dapagliflozin, canagliflozin, or empagliflozin from 1/1/2016 to 12/10/2021. Each ARD patient was matched by age, sex, and race to a patient without ARD prescribed the same SGLT2i. Baseline demographic and clinical data, prescription dates, and adverse events were collected from electronic health records for all subjects and compared in univariable analyses. Multivariable Cox models, adjusting for clinical and demographic variables, calculated hazard ratios (HR) for adverse events, following from prescription date (index) and censoring at 1st adverse event, discontinuation/last day prescription, death, or study period end. Models were then stratified by sex.
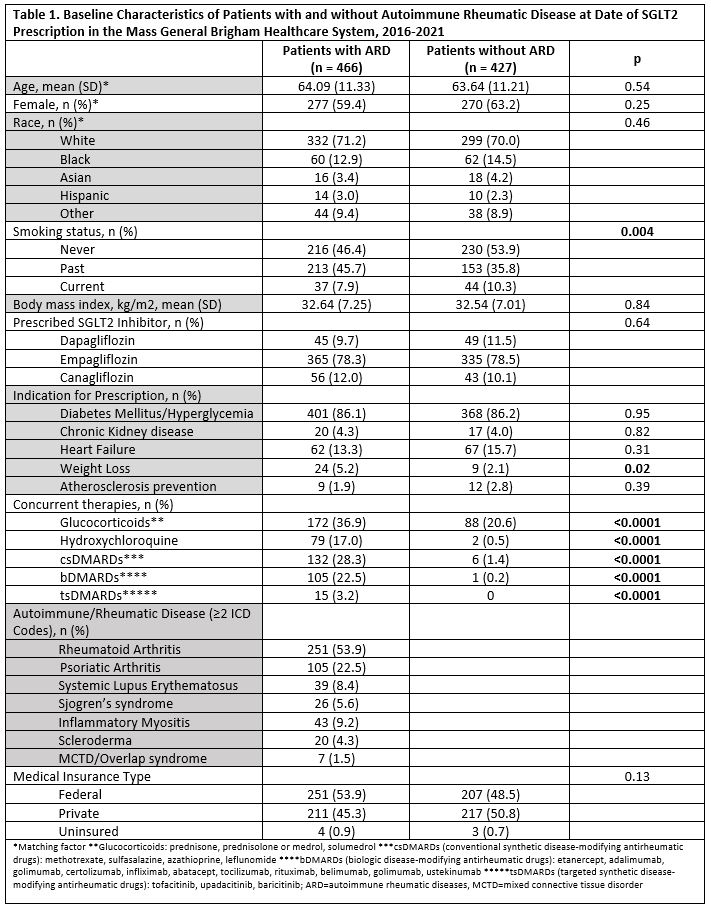
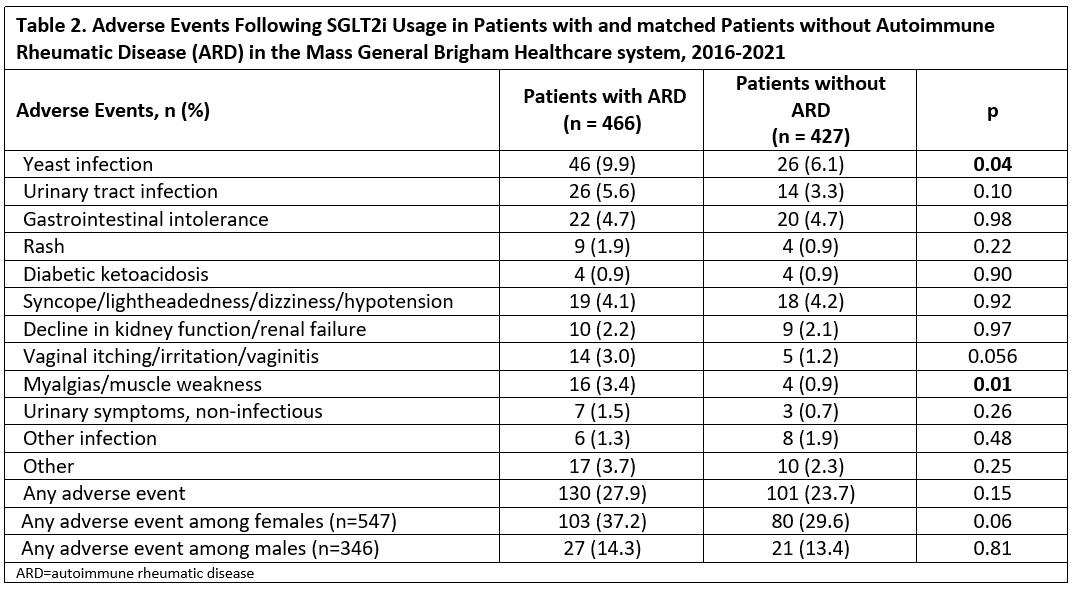
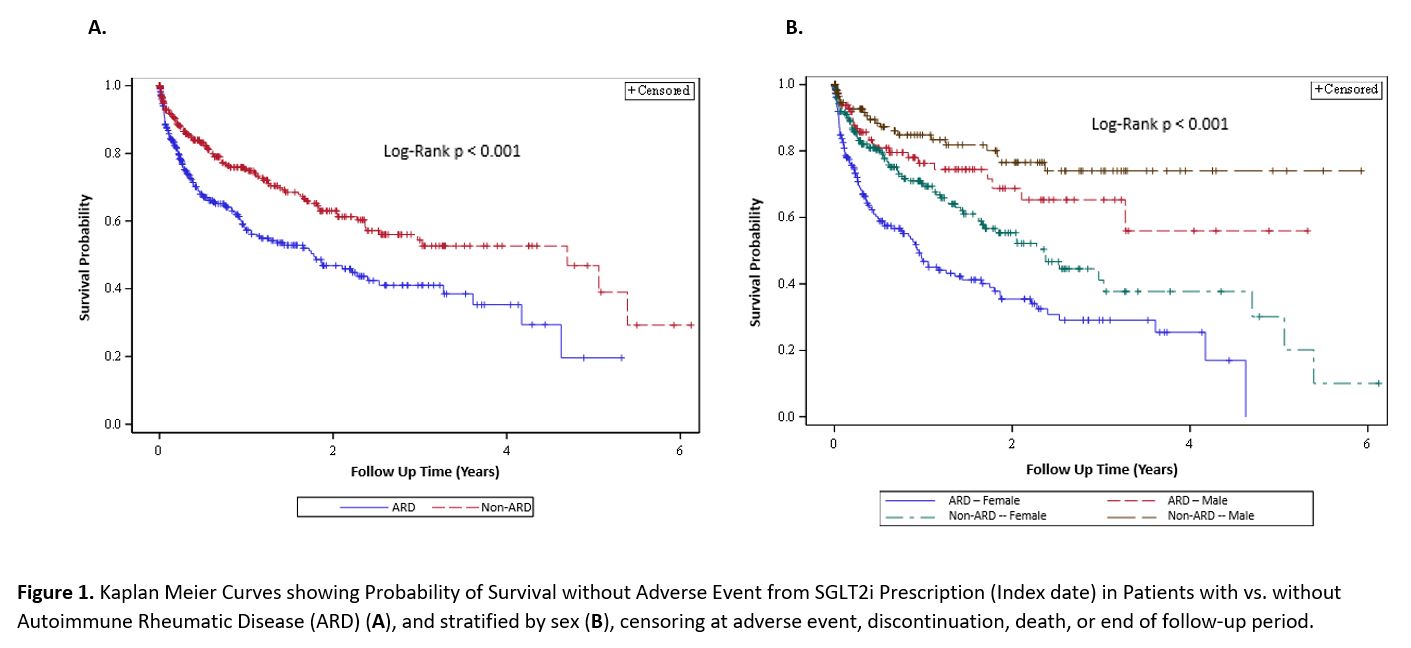
Results: We matched 519 patients with ARD to 519 patients without ARD prescribed SGLT2i: 466 and 427 in the two groups started the prescription and were studied (Table 1). The two groups were comparable (mean age 64 years; 61% female), except for past smoking, more common in patients with ARD. Empagliflozin accounted for 78% of prescriptions and T2D was the most common indication (86%). Mean hemoglobin A1c in T2D patients at index date was comparable between groups (8.08 vs 8.10 mg/dL). We identified 12 categories of adverse events and reasons for discontinuation (Table 2). Yeast infections (9.9% vs 6.1%; p 0.04) and muscular symptoms (e.g., myalgias and weakness; 3.4% vs 0.9%, p 0.01) were more frequent in ARD patients. Other adverse event categories were numerically more common in ARD patients. ARD patients also had significantly shorter SGLT2i use duration (8.7 vs 12.6 months; p< 0.0001) and time to adverse event (0.62 vs 0.96 years; p< 0.0001). We found a significant increased risk in adverse events in those with ARDs (HR 1.74 [95% CI 1.33, 2.29]), persisting upon adjustment for glucocorticoid and DMARD use (HR 1.80 [95%CI 1.34, 2.40]). Kaplan Meier curves showed separation of event-free survival over time (all log-rank p < 0.001; Figure 1). Significantly more adverse events occurred among females than males (p< 0.00001). Adverse event risk was also higher in women with ARD vs without ARD, even upon adjustment for glucocorticoid and immunosuppressant use (HR 2.05 [95% CI 1.47, 2.85]).
Conclusion: This observational study identified significant increased adverse event risk in patients with vs. without ARDs using SGLT2i. Both ARD and non-ARD female patients were more likely to have adverse events than males, with female ARD patients most affected. To our knowledge, this is the first study to consider SGLT2i adverse events in ARD vs. non-ARD patients. Further rigorous testing of safety and efficacy of SGLT2i among patients with ARDs is warranted.
To cite this abstract in AMA style:
Oakes E, Ellrodt J, Choi M, Yee J, Guan H, Costenbader K. Adverse Events Among Patients with and Without Autoimmune Rheumatic Disease Prescribed SGLT2 Inhibitors [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/adverse-events-among-patients-with-and-without-autoimmune-rheumatic-disease-prescribed-sglt2-inhibitors/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adverse-events-among-patients-with-and-without-autoimmune-rheumatic-disease-prescribed-sglt2-inhibitors/