Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Tanezumab (TNZ) has been shown to be efficacious for pain and function in patients with hip and knee osteoarthritis (OA). Unexpected reports of adverse events described as osteonecrosis (ON) developed in patients enrolled in clinical trials in the TNZ development program. These reports led the FDA to impose a temporary clinical hold on the program.
Methods: Patients with reported adverse events of ON (N = 87) and reported total joint replacement (TJR) unrelated to ON (N = 299) were identified. Source documents were requested from site investigators; documents from 87 (100%) and 162 (54.2%) of patients with ON or TJR unrelated to ON, respectively, were obtained. All available source documents were reviewed by an independent adjudication committee (IAC) comprised of 3 rheumatologists, 1 orthopedic surgeon and 1 bone pathologist who were blinded to treatment assignment. Consensus was reached on all but 7 cases.
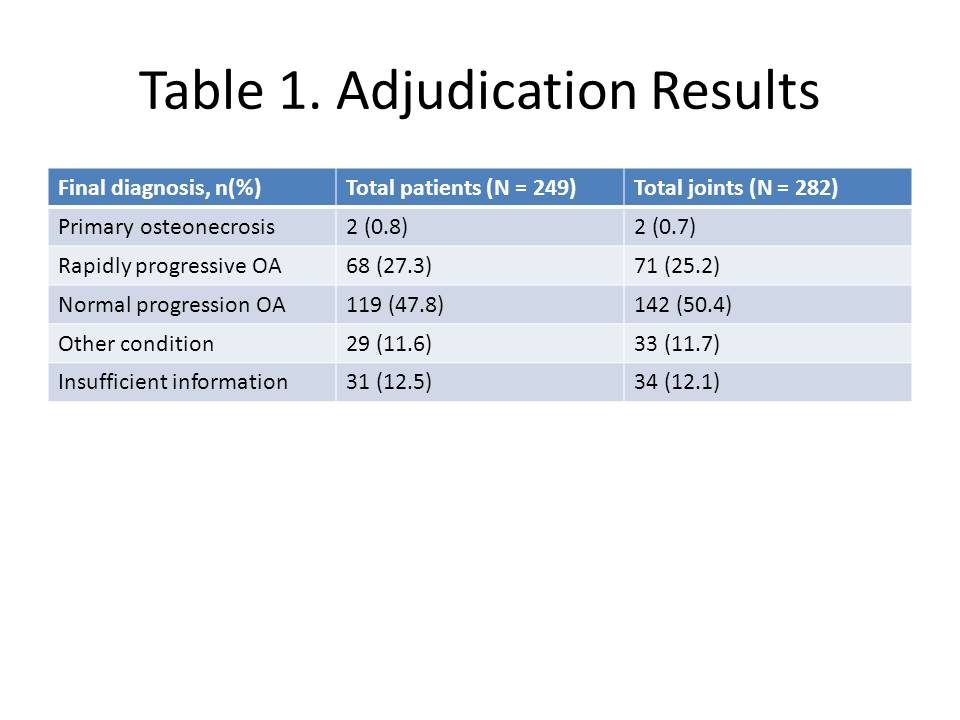
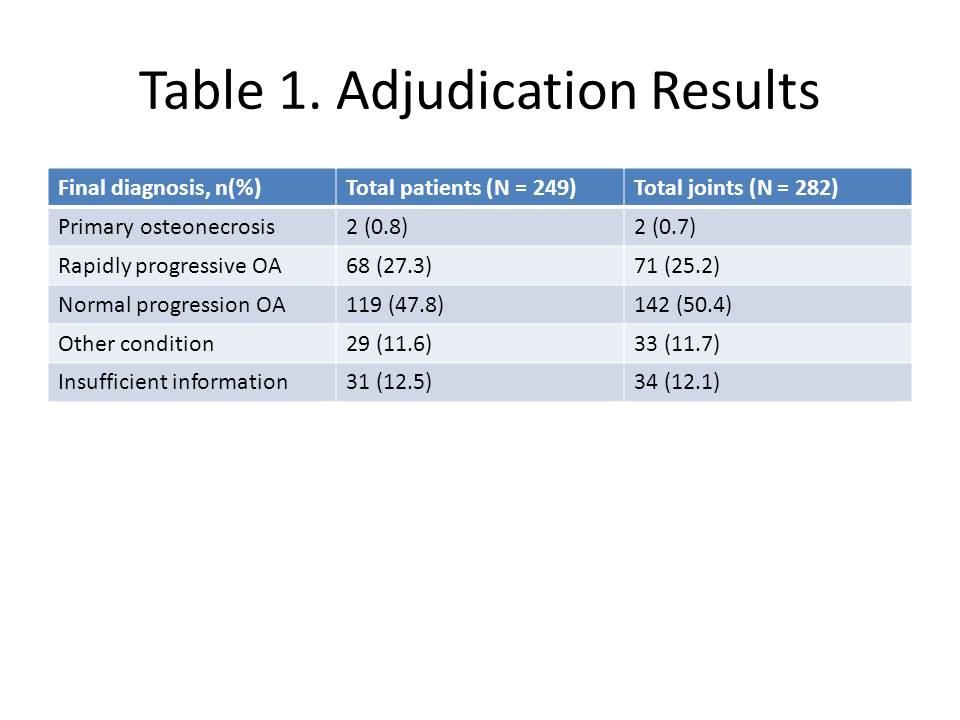
Results: The IAC classified the 249 cases into four categories: Primary ON; Rapidly progressive OA (RPOA; type 1 = loss of joint space width ≥ 1 mm over approx. 1 year, or type 2 = abnormal loss/destruction of bone uncommon for end-stage OA); Normal progression of OA (NPOA); Not enough information to distinguish between RPOA and NPOA; Other joint condition/diagnosis; or Not enough information to distinguish between primary ON, worsening OA or another diagnosis. Results of the adjudication are shown in Table 1. Of the 87 patients with an adverse event reported as ON, only 2 were adjudicated with primary ON; 34 (39.1%) were adjudicated with RPOA while the majority of others were adjudicated with NPOA or subchondral insufficiency fracture. The remaining 34 patients adjudicated with RPOA had a TJR unrelated to an adverse event of ON.
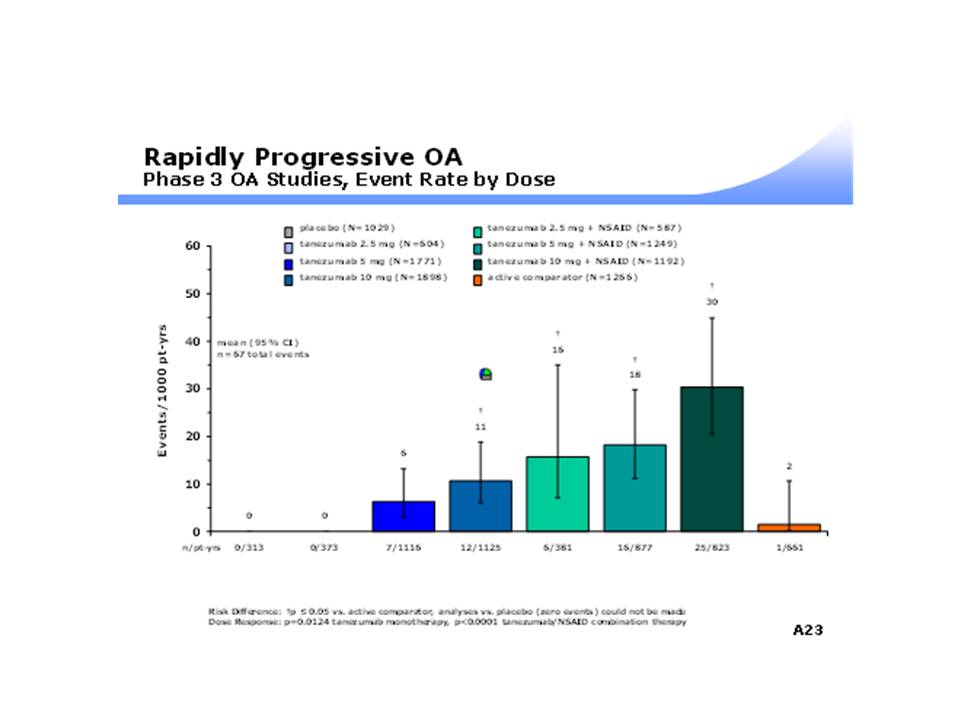
There was a significant dose-response relationship between incidence of RPOA and increasing dose of TNZ when given as monotherapy (0, 6 and 11 per 1000 person-years at doses of 2.5, 5 and 10 mg, respectively [P = 0.0124]) (see Figure 1). There was also an increased incidence of RPOA when TNZ was given in combination with NSAIDs as compared to montherapy (P < 0.001). Incidence was increased in all combination treatments compared to placebo, TZB monotherapy, and active comparator treatments. The highest rate of RPOA occurred in the TNZ 10 mg in combination with NSAIDs group (30 per 1000 person-years).
Conclusion: The rate of RPOA was greatest in patients who received TNZ with NSAIDs or TNZ alone at doses >= 10 mg. The mechanism for this association remains unclear. These results support the continued study of TNZ alone at doses below 10 mg for treatment of OA.
Disclosure:
M. C. Hochberg,
Abbott Laboratories, Astra-Zeneca, Bioiberica S.A., Eli Lilly Inc., Genentech/Roche, Merck Inc., Novartis Pharma A.G., Pfizer Inc., Stryker LLC, Xoma.,
5;
S. B. Abramson,
Pfizer Inc.,
5;
D. S. Hungerford,
Pfizer Inc.,
5;
E. McCarthy,
Pfizer Inc.,
5;
E. P. Vignon,
Pfizer Inc.,
5;
M. D. Smith,
Pfizer Inc,
1,
Pfizer Inc,
3;
L. Tive,
Pfizer Inc.,
3;
K. M. Verburg,
Pfizer Inc,
1,
Pfizer Inc,
3;
C. R. West,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adjudication-of-reported-serious-adverse-joint-events-in-the-tanezumab-clinical-development-program/