Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Early diagnosis coupled with early intensive treatment strategies have led to marked improvements in rheumatoid arthritis (RA) patient outcomes. Despite improved access to numerous effective RA drug therapies, patient adherence can be suboptimal. The objective of the present study was to describe adherence to early DMARD strategies and associations with disease activity in “real-world” patients under routine care.
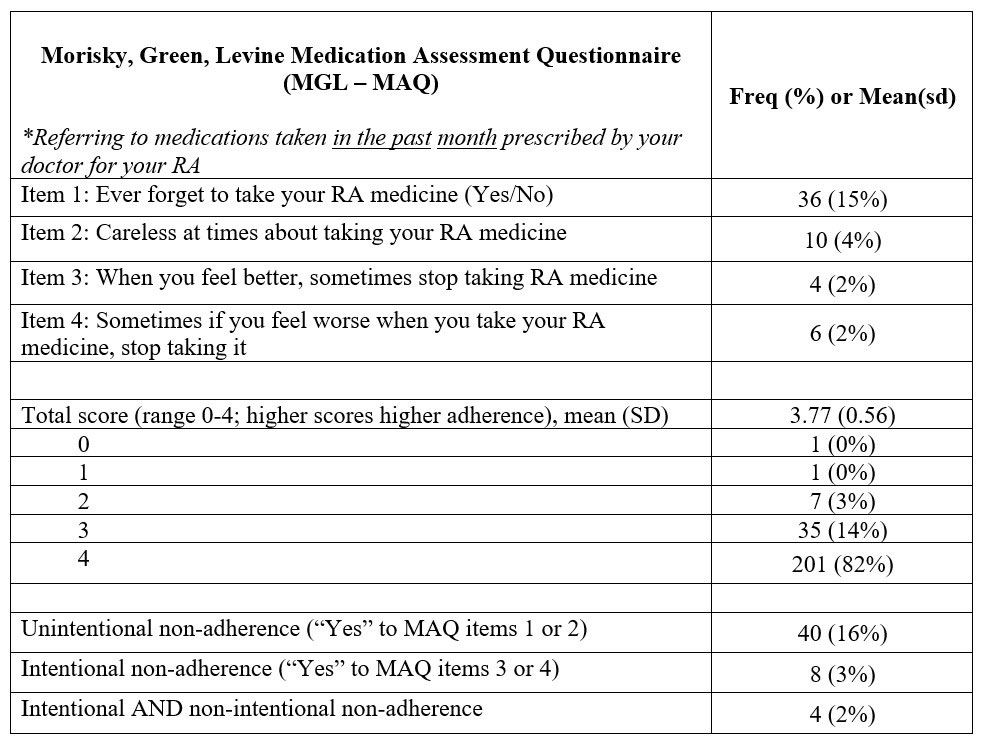
Methods: We analyzed baseline, 6- and 12-month data from the Canadian Early Arthritis Cohort (CATCH) study collected between 01-2017 and 03-2022 when a measure of medication adherence was added to the study protocol. CATCH is a prospective multi-center study of early RA patients (symptoms < 1 year; 81% fulfilling RA criteria at enrolment) diagnosed and treated in rheumatology clinics across Canada. Participants underwent detailed RA clinical assessments and completed sociodemographic and patient-reported outcome measures including the well-validated 4-item Morisky, Greene, Levine Medication Assessment Questionnaire (MGL-MAQ; range 0-low to 4-high adherence) referring to adherence to medications taken in the past month. The MGL-MAQ has also been used to classify reasons for non-adherence as unintentional (i.e., forgetting or carelessness with taking medication) and intentional (i.e., stop taking medication when feeling good or when medication makes one feel bad). Level of adherence (High MGL-MAQ =4 vs. low/moderate < 4) and reasons for non-adherence to RA treatment at 6-months follow-up were summarized using descriptive statistics. Associations between early medication adherence with CDAI disease activity as well as low disease activity/remission (LDA/RED) at 12-months were estimated with multivariable linear and logistic regression, respectively, adjusted for age, sex and SES.
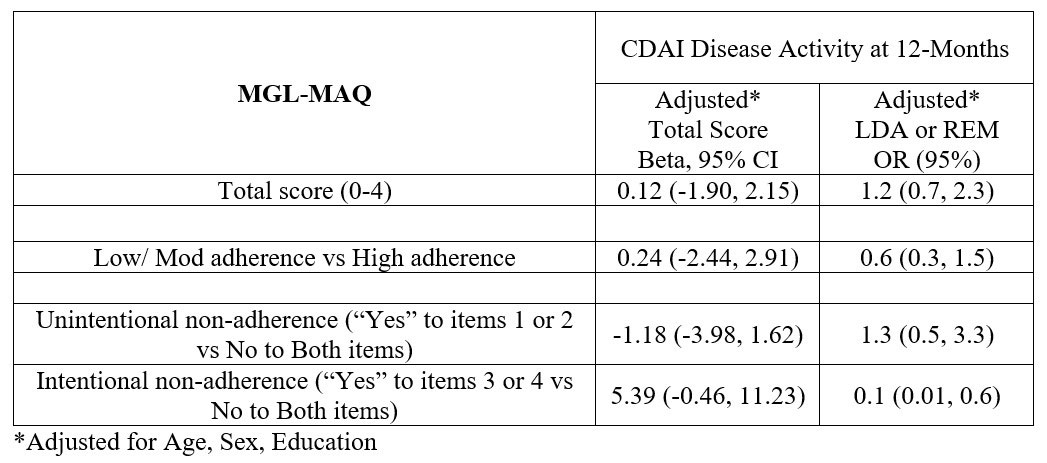
Results: The study included 245 early RA (ERA) patients, 168 (69%) were female, mean(sd) age was 57 (14), symptom duration was 5 (3) months and CDAI disease activity was high 27 (13). At enrolment, 54% of patients were treatment naïve, most initiated csDMARDs (91%) and were commonly treated with MTX (81%) over the first 3-months of RA treatment. Overall, 82% of ERA patients reported high adherence to their RA treatment at 6-months (Table 1). Amongst those reporting low/moderate adherence, 82% reported unintentional non-adherence only, 9% intentional non-adherence only and 9% both. Results of multivariable regression suggested that intentional non-adherence may be associated with higher CDAI scores and lower likelihood of LDA/REM at 12-months (Table 2).
Conclusion: In this real-world patient study embedded in routine care, approximately 1/6 of RA patients reported low/moderate adherence to their RA treatment within 6 months. Unintentional reasons for non-adherence were more frequently reported then intentional, though regression analyses suggested that intentional non-adherence may be especially associated with a lower likelihood of reaching early treatment targets. Future work in large prospective studies should aim to assess intentional non-adherence over longer follow up periods and develop targeted adherence strategies in order to optimize RA treatment outcomes.
To cite this abstract in AMA style:
Jiang Y, schieir o, Valois M, Bartlett S, Bessette L, Boire G, Hazlewood G, Hitchon C, Keystone E, Tin D, Thorne C, Bykerk V, Pope J, (CATCH) Investigators C. Adherence to Early DMARD Strategies in Newly Diagnosed RA Patients Seen in Routine Care: Results from the Canadian Early Arthritis Cohort Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/adherence-to-early-dmard-strategies-in-newly-diagnosed-ra-patients-seen-in-routine-care-results-from-the-canadian-early-arthritis-cohort-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adherence-to-early-dmard-strategies-in-newly-diagnosed-ra-patients-seen-in-routine-care-results-from-the-canadian-early-arthritis-cohort-study/