Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Most immunosuppressed patients with chronic inflammatory diseases (CID) mount total anti-Spike (S) IgG responses following vaccination with mRNA-based SARS-CoV-2 vaccines. Less is known, however, about cross-variant neutralization potential, an immune correlate of protection that highly associates with efficacy of vaccination. Here, we describe how an additional dose of SARS-CoV-2 mRNA vaccination influences both total anti-S IgG and neutralizing titers to variants of concern.
Methods: The COVID-19 Vaccine Responses in Patients with Autoimmune Disease (COVaRiPAD) study is a prospective assessment of mRNA-based vaccine immunogenicity and reactogenicity in patients with CID. A total of 340 adults with CIDs were enrolled, with 81 participants having samples available at both 5 months after completion of the initial series and 4 weeks after the third dose. Serum anti-SARS-CoV-2 spike (S) IgG+ binding and neutralizing antibody titers to vesicular stomatitis virus pseudotyped with S protein from D614G (common), (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), P.1 (Gamma), and B.1.1.519 subvariant (Omicron) were quantified.
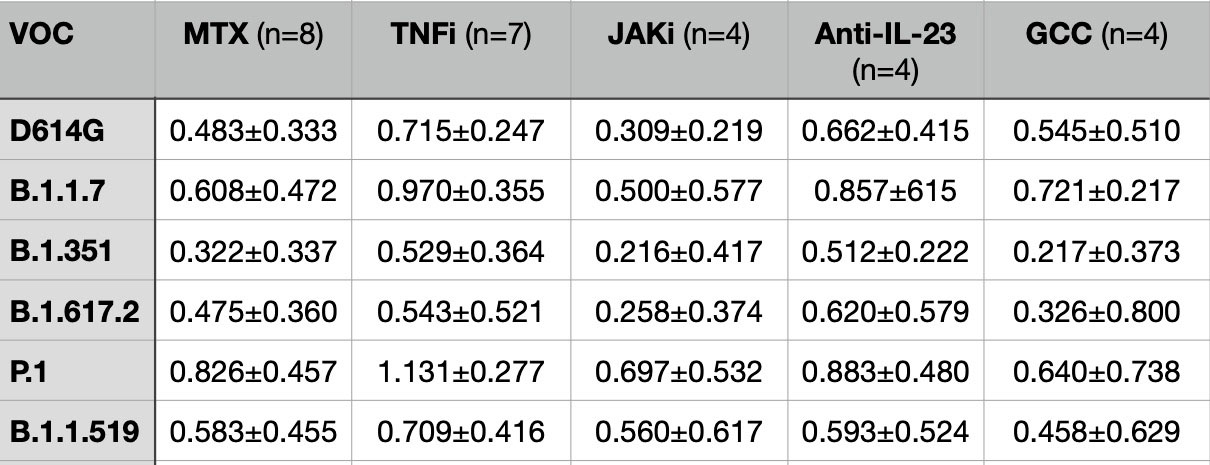
Results: When examining the effect of a third dose of vaccine, total anti-S IgG titers increased >100-fold for most classes with exception of those on B cell depleting therapies. The additional dose did increase neutralization titers to all variants tested in most immunosuppressive classes with n ≥ 4 (Table 2), including 3- to 9-fold increases in neutralization against Omicron.
Conclusion: Reassuringly, immunosuppressed patients with CID mounted improved total anti-S IgG and neutralizing antibody titers to variants of concern broadly across various immunosuppressive classes. This highlights the necessity to provide additional doses beyond the initial series, which likely will extend to doses beyond the third dose.
To cite this abstract in AMA style:
Paley M, Deepak P, Kim W, Yang M, Chandrasekaran V, Oliva Escudero G, Huang K, Liu Z, McMorrow L, Thapa M, Ciorba M, Matloubian M, Gensler L, Nakamura M, Whelan S, Buchser W, Ellebedy A, Kim A. Additional Dose of SARS-CoV-2 Vaccine Improves Cross-Variant Neutralization Titers in Immunosuppressed Patients with Chronic Inflammatory Disease [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/additional-dose-of-sars-cov-2-vaccine-improves-cross-variant-neutralization-titers-in-immunosuppressed-patients-with-chronic-inflammatory-disease/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/additional-dose-of-sars-cov-2-vaccine-improves-cross-variant-neutralization-titers-in-immunosuppressed-patients-with-chronic-inflammatory-disease/